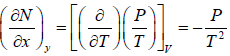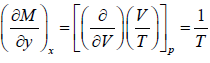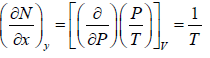Basics of Thermodynamics | Kinetic Theory & Thermodynamics - Physics PDF Download
Mathematical Formulations of Thermodynamics
Y = Y(X1, X2 ,......Xn)
then differential dY is said to be exact and one can write
dY = 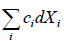
dY = c1dX1+ c2dX2 + ........
dY = 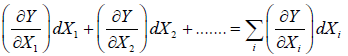
and its corresponding ci Xi are said to be conjugate to each other.
It is given that df = Adx + Bdy + Cdz If f is perfect differential then
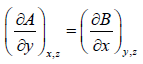
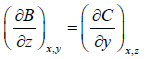
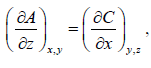
Some Important Formulas
1. 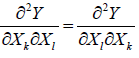
2. 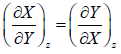
3. 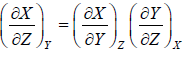
4. 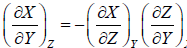
5. 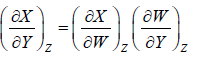
6. dX = 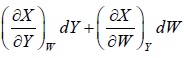
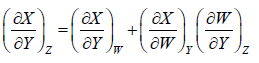
System
A system can be any object, region of space etc, selected for study and set apart (mentally) from everything else. All other thing except system is identified as surrounding. The system of interest in thermodynamics are finite and macroscopic rather than microscopic. The imaginary envelope which encloses a system and separate it from its surrounding is called the boundary of the system.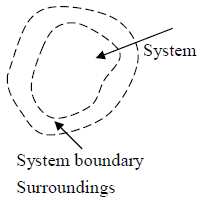
Open system:
When Heat and Mass flowing across the boundary the system is identified as open system.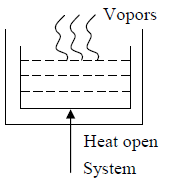
For boiling water without a lid, heat is transferred into the air and at same time steam (which is matter) also mixed into the air.
Closed system:
When Heat can be exchanged across the boundary but Mass can not flow across the boundary the system is identified as closed system. Example is electric bulb.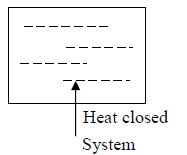
Isolated System:
It cannot exchange either matter or energy with the surrounding. Example: Thermal flask, Universe.
Thermodynamical State
A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to represent the system.
Thermodynamic state is the macroscopic condition of a thermodynamic system as described by its particular thermodynamic parameter, such as temperature (T), pressure
(P), volume (V) and density(ρ).
State function
State function also called “State variable” are those thermodynamic variables that describe the momentary condition of thermodynamic system. For a continuous process,
such variable are exact differential also fully determined by their initial and final
thermodynamic states, which is also known as thermodynamic property.
A thermodynamic system can be completely defined when certain parameters (State
Variable) are completely defined. These system parameters are called thermodynamic
properties. The position (state) of a system can be shown on a property diagram.
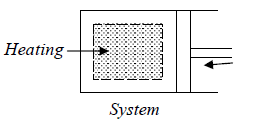
For example, the system comprises of Ideal gas confined in a cylinder with a frictionless
piston. When the system gets heat the gas expands and piston moves out i.e., pressure
will change from P1 tp P2 and volume expands from V1 to V2.
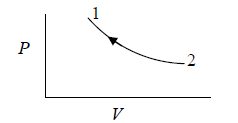
Property diagram of Ideal gas in P - V diagram when it is heating.
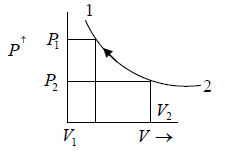
Property Diagram of Ideal gas when it is cooling. If we cool the system the piston will
move in opposite direction and gas is compressed .
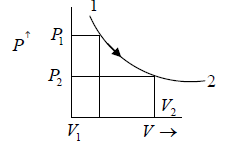
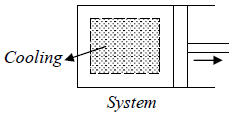
When piston is compressed then pressure increased from P2 to P1 and volume decrease from V2 to V1. and V here are properties of system parameters.
Thermodynamic properties are macroscopic properties, they are mainly
Pressure (P) : Pressure is the force applied perpendicular to the surface of an object
per unit area over which that force is distributed. The SI unit of pressure N /m2
Volume (V) : Volume is the quantity of three-dimensional space enclosed by a closed
surface, for example, the space that a substance or shape occupies or contains. Volume is
often quantified numerically using the SI derived unit, the cubic meter. The volume of a
container is generally understood to be the capacity of the container; i.e., the amount
of fluid (gas or liquid) that the container could hold
Temperature (T): Temperature is a physical quantity expressing hot and cold. It is a proportional measure of the average kinetic energy of the random motions of the constituent particles of matter (such as atoms and molecules) in a system.
Entropy (S) : Entropy is the measure of randomness of a system.
If heat Q is transferred to a system at temperature T , entropy increases and if Heat is
transferred from body then entropy decreases.
The change in entropy ∫dS = 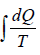 or S2 - S2 = Q/T
or S2 - S2 = Q/T
dS = dQ/T is used for reversible process But for irreversible process dS > dQ/T.
Internal Energy (U) : Internal energy of a system which comprises of Potential
energy, kinetic energy, vibration energy, rotational energy etc. of the system particles.
For an ideal gas internal energy is only function of temperature,U = f (T)
Internal energy will also defined Specific heat at constant volume which is given by
Cv = 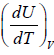
Hence internal energy is point function then their change are measured by difference in initial and final state only.
ΔU = U2 - U1. For close path 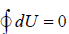
Enthalpy (H) - Enthalpy of a gas o a fluid is defined as H = U + PV where U is Specific heat at constant pressure Cp = dH/dT.
ΔH = H1 - H2
Change in enthalpy H2 - H1 = (U2 + P2 + V2) - (U1 - P1 - V1) of fluid.
For ideal gas U= f(T) and PV = RT
So for Ideal Gas, enthalpy is also only function of temperature i.e., H = f(T)
Helmtloltz Free Energy (F) - The Helmholtz free energy is given by F = U - TS is the measure of potential between any two points during a process (Chemical or flow) which tells about possibility of the process on forward direction. The Helmholtz free energy is measurement of Available energy for work.
Gibbs Energy (G) - Gibbs energy determines the spontaneity of a process.
G = H - TS
ΔG = ΔH - TΔS (For isothermal) process of
ΔG > 0 Non spontaneous ⇒ ΔH > TΔS or ΔH/T > ΔS
ΔG < 0 Spontaneous ⇒ or ΔH/T < ΔS
ΔG = 0 Spontaneous or ΔH/T = ΔS
Mathematical condition for state function
If f (x, y) is state function (thermodynamic property) or path independent then with variable x and y then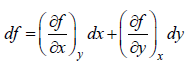 ⇒ df Md x Mdy where M =
⇒ df Md x Mdy where M = 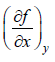 N =
N = 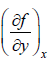
If f (x, y) is exact differential, then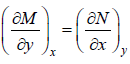
Example 1: If f(T,V) = 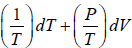 then prove that f(T,V) is path independent or State function or thermodynamic property.
then prove that f(T,V) is path independent or State function or thermodynamic property.
Here M = 1/T, N = P/T and x = T and y = V
= 0 and
Since,, the given expression is not an exact differential, and hence not a thermodynamic property.
Example 2: If function f (P,V) is defined as f (P,V) = 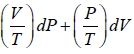 then prove that
then prove that
f (P,V) is thermodynamic property or State function.
f (P,V) = 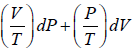
M = V/T = R/p and N = p/T = R/V x = P and y = V
Since,, the given expression is an exact differential, and hence thermodynamic property or state function
Intensive and extensive properties
Intensive properties: It is a physical property of a system that does not depend on the system size or the amount of material in system. It is scale invariant.
Example: Chemical potential, density, viscosity, resistivity, specific heat capacity, pressure, elasticity, magnetization, velocity, acceleration, temperatures, etc.
Extensive Properties: It is additive for independent non-interacting subsystem. It is directly proportional to the amount of material in the system.
Example: Energy, Entropy, Gibbs energy, mass momentum, volume, change, weight,
Note: If f and g are arbitrary intensive variable, then 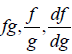 and f + g will also be
and f + g will also be
intensive variables.
If F and G are two arbitrary extensive variables, then F + G will be an extensive variable, but F/G and dF/dG will be intensive.
If F is extensive variable and f is intensive variable, the dF, F/f and dF/df will be extensive variables.
Reversible and irreversible process
The process in which the system and surroundings can be restored to the initial state from the final state without producing any changes in the thermodynamics properties of the universe is called a reversible process, otherwise the process is irreversible. dS = dQ/T
is used for reversible process But for irreversible process dS > dQ/T .
Quasi static process
A quasi-static process is a thermodynamic process slow enough that system to remain in internal equilibrium. The volume of a system changes at a slow rate enough to allow the pressure to remain uniform and constant throughout the system. Any reversible process is also a quasi-static one, but the converse is not true.
Thermodynamic equilibrium
In thermodynamic equilibrium there are no net macroscopic flows of matter or of energy, either within a system or between systems. In thermodynamic equilibrium the system will have uniform temperature.
Equation of State
In thermodynamics, an equation of state is a thermodynamic equation relating state variables which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature (P,V,T) , or internal energy.
Equation of State for Ideal Gas
The Ideal gas law in the equation of state of a hypothetical Ideal gas. It is derived from kinetic theory and satisfies the Boyle’s and Charles’ law.
The state of an amount (number of mole n ) of ideal gas is determined by its pressure
(P) , volume (V) and temperature (T).
The Ideal gas equation of state for n mole is given by PV = nRT , where R is gas
constant and is equal to 8.314 J.K-1mole-1
Equation of state for Real gases (van der Waal equation)
The state of an amount of Real gas (number of mole n ) is determined by its pressure (P) , volume (V) and temperature (T).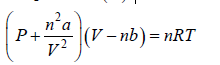
|
6 videos|20 docs|32 tests
|
FAQs on Basics of Thermodynamics - Kinetic Theory & Thermodynamics - Physics
| 1. What is the mathematical formulation of thermodynamics? |  |
| 2. What is the difference between intensive and extensive properties in thermodynamics? |  |
| 3. What is a reversible process in thermodynamics? |  |
| 4. What is the meaning of thermodynamic equilibrium? |  |
| 5. What is an equation of state in thermodynamics? |  |