Bank Exams Exam > Bank Exams Notes > IBPS PO Prelims & Mains Preparation > Branches of Science, Elements and Compounds
Branches of Science, Elements and Compounds | IBPS PO Prelims & Mains Preparation - Bank Exams PDF Download
Different Branches of Science
Branch ➜ Concerning Field
- Aeronautics ➜ Science of flight of aeroplanes.
- Astronomy ➜ Study of heavenly bodies.
- Agronomy ➜ Science dealing with crop plants.
- Angiology ➜ Deals with the study of the blood vascular system.
- Anthology ➜ Study of flowers.
- Anthropology ➜ Study of apse and man.
- Apiculture ➜ Honey industry (Bee Keeping)
- Araneology ➜ Study of spiders.
- Batracology ➜ Study of frogs.
- Biochemistry ➜ deals with the study of chemical reactions in relation to life activities.
- Biotechnology ➜ Deals with the use of micro-organisms in commercial processes for producing fine chemicals such as drugs, vaccines, hormones, etc. on a large scale.
- Cardiology ➜ Study of heart.
- Craniology ➜ Study of skulls.
- Cryptography ➜ Study of secret writing.
- Cryogenics ➜ Study concerning the application and uses of very low temperature.
- Cytology ➜ Study of cells.
- Dermatology ➜ Study of skin.
- Ecology ➜ The study of the relationship between organisms and the environment.
- Entomology ➜ Study of insects.
- Etiology ➜ Study of the cause of disease.
- Eugenics ➜ Study of improvement of the human race by applying laws of heredity. It is related to future generations.
- Evolution ➜ Deals with the study of the origin of new from old.
- Exobiology ➜ Deals with life or possibilities of life beyond the earth.
- Floriculture ➜ Study of flower yielding plants.
- Geology ➜ Study of condition and structure of the earth.
- Genetics ➜ Study of heredity and variations.
- Gerontology ➜ Study of female reproductive organs.
- Horticulture ➜ Study of garden cultivation.
- Haematology ➜ Study of blood.
- Herpetology ➜ Study of the liver.
- Iconography ➜ Teaching by pictures and models.
- Immunology ➜ science which deals with the study of resistance of organisms against infection.
- Jurisprudence ➜ Science of law.
- Kalology ➜ Study of human beauty.
- Lexicography ➜ Compiling of a dictionary.
- Mycology ➜ Study of fungi.
- Myology ➜ Study of muscles.
- Nephrology ➜ Study of kidneys.
- Neurology ➜ Study of the nervous system.
- Numismatics ➜ Study of coins and medals.
- Obstetrics ➜ Branch of medicine dealing with pregnancy.
- Oneirology ➜ Study of dreams.
- Ophthalmology ➜ Study of eyes.
- Ornithology ➜ Study of birds.
- Osteology ➜ Study of bones.
- Palaeontology ➜ Study of fossils.
- Philately ➜ Stamp collecting.
- Philology ➜ Study of languages.
- Phonetics ➜ Concerning the sounds of a spoken language.
- Physiography ➜ Nature phenomenon.
- Pedology ➜ Study of soils.
- Pathology ➜ Study of disease-causing organisms.
- Phycology ➜ Study of algae.
- Physiology ➜ Science deals with the study of functions of various parts of organisms.
- Pisciculture ➜ Study of fish.
- Pomology ➜ Study of fruits.
- Seismology ➜ Study of earthquakes.
- Sericulture ➜ Silk industry (culture of silk moth and pupa).
- Serpentology ➜ Study of snakes.
- Telepathy ➜ communication between two minds at a distance with the help of emotions.
- Taxonomy ➜ Study of classification of organisms.
- Virology ➜ Study of the virus.
Question for Branches of Science, Elements and CompoundsTry yourself:The science of sound is dealt by
View Solution
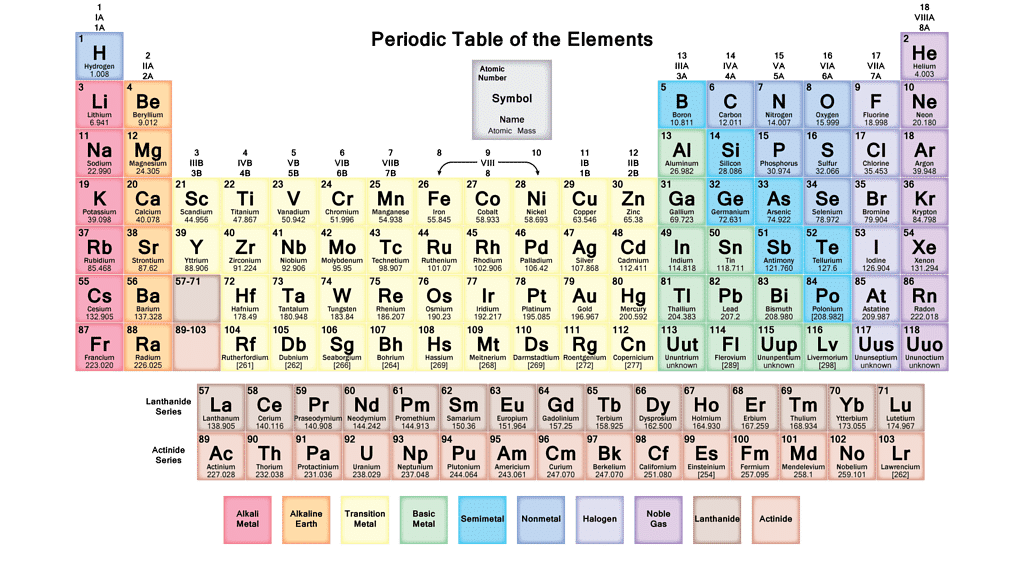
Elements, Symbols And Atomic Numbers
Element(Symbol) ➜ Atomic Number
- Hydrogen (H) ➜ 1
- Helium (He) ➜ 2
- Lithium (Li) ➜ 3
- Beryllium (Be) ➜ 4
- Boron (B) ➜ 5
- Carbon (C) ➜ 6
- Nitrogen (N) ➜ 7
- Oxygen (O) ➜ 8
- Fluorine (F) ➜ 9
- Neon (Ne) ➜ 10
- Sodium (Natrium) (Na) ➜ 11
- Magnesium (Mg) ➜ 12
- Aluminum (Al) ➜ 13
- Silicon (Si) ➜ 14
- Phosphorous (P) ➜ 15
- Sulphur (S) ➜ 16
- Chlorine (Cl) ➜ 17
- Argon (Ar) ➜ 18
- Potassium (Kalium) (K) ➜ 19
- Calcium (Ca) ➜ 20
- Titanium (Ti) ➜ 22
- Vanadium (V) ➜ 23
- Chromium (Cr) ➜ 24
- Manganese (Mn) ➜ 25
- Iron (Ferrum) (Fe) ➜ 26
- Cobalt (Co) ➜ 27
- Nickel (Ni) ➜ 28
- Copper (Cuprum) (Cu) ➜ 29
- Zinc (Zn) ➜ 30
- Germanium (Ge) ➜ 32
- Bromine (Br) ➜ 35
- Krypton (Kr) ➜ 36
- Zirconium (Zr) ➜ 40
- Silver (Ag) ➜ 47
- Tin (Stannum) (Sn) ➜ 50
- Antimony (Stabnium) (Sb) ➜ 51
- Iodine (I) ➜ 53
- Barium (Ba) ➜ 56
- Gold (Aurum) (Au) ➜ 79
- Mercury (Hydragerm) (Hg) ➜ 80
- Lead (Plumbum) (Pb) ➜ 82
- Bismuth (Bi) ➜ 83
- Radium (Ra) ➜ 88
- Thorium (Th) ➜ 90
- Uranium (U) ➜ 92
- Plutonium (Pu) ➜ 94
- Curium (Cm) ➜ 96Question for Branches of Science, Elements and CompoundsTry yourself:What is atomic number for Sodium (Natrium)(Na)?View Solution
Common and Chemical Names of Some Compounds
Common Name ➜ Chemical Name (Chemical Formula)
- Dry ice ➜ Solid Carbon Dioxide (CO2)
- Slaked Lime ➜ Calcium Hydroxide (Ca(OH)2)
- Bleaching Powder ➜ Calcium Oxychloride (CaOCI2)
- Nausadar ➜ Ammonium Chloride (NH4CI)
- Caustic Soda ➜ Sodium Hydroxide (NaOH)
- Rock Salt ➜ Sodium Chloride (NaCI)
- Caustic Potash ➜ Potassium Hydroxide (KOH)
- Potash Alum ➜ Potassium Aluminium Sulphate (K2SO4.AI2(SO4)3.24H2O)
- Epsom ➜ Magnesium Sulphate (MgSO4.7H2O)
- Quick Lime ➜ Calcium Oxide (CaO)
- Plaster of Paris ➜ Calcium Sulphate Hemihydrate (CaSO4)1/2 H2O
- Gypsum ➜ Calcium Sulphate (CaSO4.2H2O)
- Green Vitriol ➜ Ferrous Sulphate (FeSO4.7H2O)
- Mohr’s Salt ➜ Ammonium Ferrous Sulphate (FeSO4 (NH4)2SO4.6H2O)
- Blue Vitriol ➜ Copper Sulphate (CuSO4.5H2O)
- White Vitriol ➜ Zinc Sulphate (ZnSO4.7H2O)
- Marsh Gas ➜ Methane (CH4)
- Vinegar ➜ Acetic Acid (CH3COOH)
- Potash Ash ➜ Potassium Carbonate (K2CO3)
- Hypo ➜ Sodium Thiosulphate (Na2S2O3.5H2O)
- Baking Powder ➜ Sodium Bicarbonate (NaHCO3)
- Washing Soda ➜ Sodium Carbonate (Na2CO3.10H2O)
- Magnesia ➜ Magnesium Oxide (MgO)
- Chalk (Marble) ➜ Calcium Carbonate (CaCO3)
- Lunar Caustic ➜ Silver Nitrate (AgNO3)
- Laughing Gas ➜ Nitrous Oxide (N2O)
- Chloroform ➜ Trichloro Methane (CHCI3)
- Vermelium ➜ Mercuric Sulphide (HgS)
- Borax ➜ Borax (Na2B4O7.10H2O)
- Alcohol ➜ Ethyl Alcohol (C2H5OH)
- Sugar ➜ Sucrose (C12H22O11)
- Heavy Water ➜ Deuterium Oxide (D2O)
- Globar’s Salt ➜ Sodium Sulphate (Na2SO4.10H2O)
- T.N.T. ➜ Tri Nitrotoluene (C5H2CH3(NO2)3)
- Calomel ➜ Mercurous Chloride (Hgci)
- Sand ➜ Silicon Oxide (SiO2)
Question for Branches of Science, Elements and CompoundsTry yourself:What is chemicl name for sugar?
View Solution
The document Branches of Science, Elements and Compounds | IBPS PO Prelims & Mains Preparation - Bank Exams is a part of the Bank Exams Course IBPS PO Prelims & Mains Preparation.
All you need of Bank Exams at this link: Bank Exams
|
541 videos|683 docs|263 tests
|
FAQs on Branches of Science, Elements and Compounds - IBPS PO Prelims & Mains Preparation - Bank Exams
| 1. What are the different branches of science? |  |
Ans. The different branches of science are:
1. Physics - study of matter, energy, and their interactions.
2. Chemistry - study of the composition, properties, and interactions of matter.
3. Biology - study of living organisms and their interactions with the environment.
4. Geology - study of the Earth's structure, processes, and history.
5. Astronomy - study of the universe beyond the Earth's atmosphere.
| 2. What are elements, symbols, and atomic numbers? |  |
Ans. Elements are substances that cannot be broken down into simpler substances by chemical means. Each element has a unique symbol, consisting of one or two letters, which is used to represent it. The atomic number of an element is the number of protons in the nucleus of an atom of that element. It is unique to each element and determines its place in the periodic table.
| 3. What are the common and chemical names of some compounds? |  |
Ans.
1. Water - common name, chemical name is H2O.
2. Sodium chloride - common name, chemical name is NaCl.
3. Carbon dioxide - common name, chemical name is CO2.
4. Ammonia - common name, chemical name is NH3.
5. Hydrogen peroxide - common name, chemical name is H2O2.
| 4. What is the importance of studying science? |  |
Ans. Studying science is important because it helps us understand the world around us. It enables us to develop new technologies, solve problems, and make informed decisions about issues like climate change, healthcare, and energy. Science also helps us understand the natural laws that govern the universe and the processes that shape our planet, from the tiniest particles to the largest galaxies.
| 5. What is the difference between a physical and a chemical change? |  |
Ans. A physical change is a change in the physical properties of a substance, such as its shape, size, or state of matter, without changing its chemical composition. Examples include melting ice, boiling water, and crushing a can. A chemical change involves a chemical reaction that changes the chemical composition of a substance. Examples include burning wood, rusting iron, and baking a cake.
|
541 videos|683 docs|263 tests
|
Download as PDF

|
Explore Courses for Bank Exams exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches