Year 11 Exam > Year 11 Notes > Physics for GCSE/IGCSE > Brownian Motion
Brownian Motion | Physics for GCSE/IGCSE - Year 11 PDF Download
The Concept of Brownian Motion
- The Kinetic Theory of Matter, asserting that all matter consists of minuscule particles, was stumbled upon almost inadvertently.
- Scottish scientist Robert Brown initially observed the erratic movement of pollen grains in water through a microscope.
- At the time, this phenomenon remained unexplained, but it was later understood to demonstrate that substances comprise particles in perpetual motion, hence the term 'kinetic.'

- Brownian motion refers to the haphazard movement of particles within a liquid or gas, induced by numerous collisions with smaller, often imperceptible particles.
- When minute particles like pollen or smoke are dispersed in a liquid or gas, their random and irregular movement can be observed under a microscope.
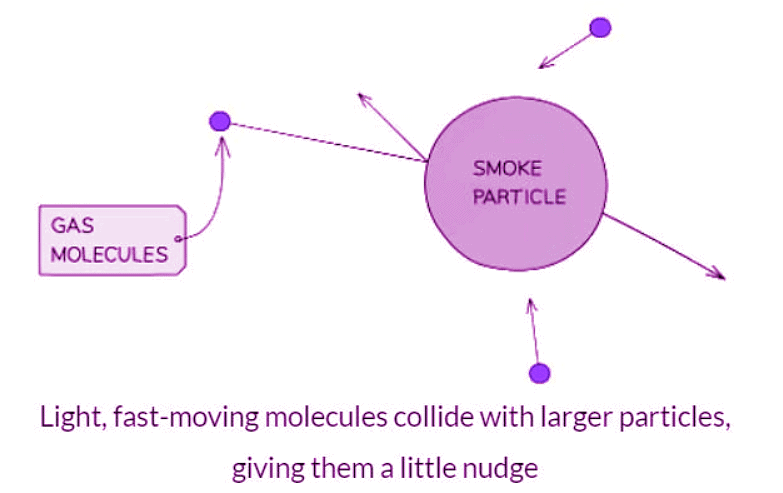
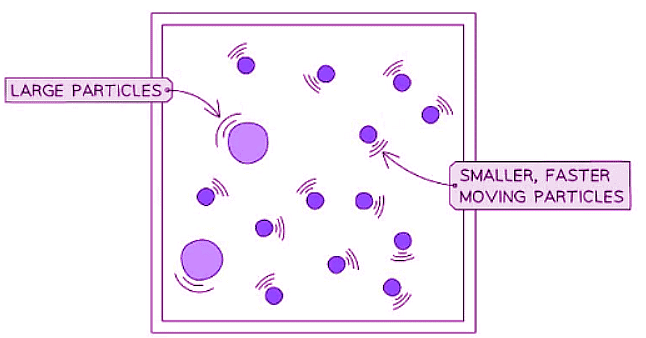
- When observing Brownian Motion, only the minute particles visible under a microscope can be detected.
- The movement of pollen or smoke particles is observable.
- Atoms and molecules of water or air, being even smaller, remain invisible.
- These small, swift-moving atoms and molecules interact with the larger microscopic particles.
- These collisions provide a slight impulse to the particles, causing their speed and direction to alter randomly with each collision.
- The existence of these light, rapidly moving atoms and molecules is deduced from the motion of the microscopic particles.
- Such deductions play a crucial role in scientific inquiry.
Question for Brownian MotionTry yourself: What is the term used to describe the random and irregular movement of particles within a liquid or gas?View Solution
The document Brownian Motion | Physics for GCSE/IGCSE - Year 11 is a part of the Year 11 Course Physics for GCSE/IGCSE.
All you need of Year 11 at this link: Year 11
|
127 videos|148 docs|35 tests
|
FAQs on Brownian Motion - Physics for GCSE/IGCSE - Year 11
| 1. What is Brownian Motion? |  |
Ans. Brownian Motion is the random movement of microscopic particles suspended in a fluid, caused by the impact of molecules of the fluid.
| 2. How does Brownian Motion relate to the Kinetic Theory of Matter? |  |
Ans. Brownian Motion is a phenomenon that supports the Kinetic Theory of Matter, which states that all matter is made up of tiny particles in constant motion.
| 3. Can you provide an overview of Brownian Motion? |  |
Ans. Brownian Motion is the continuous, random motion of particles suspended in a fluid, resulting from the collision of fluid molecules with the particles.
| 4. How does Brownian Motion occur in real-life scenarios? |  |
Ans. Brownian Motion can be observed in various everyday situations, such as the movement of smoke particles in the air or the dispersion of perfume in a room.
| 5. Why is understanding Brownian Motion important in the study of physics? |  |
Ans. Understanding Brownian Motion is crucial in physics as it provides insights into the behavior of particles at a microscopic level and helps explain various phenomena in the natural world.

|
Explore Courses for Year 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches
















