Class 9 Science Chapter 1 Case Based Questions - Matter in Our Surroundings
Q1: Read the following iformation and answer the questions that follows based on the passage and releted studied concepts.
Boiling occurs when the particles in a liquid state absorb enough energy to overcome the forces holding them together and begin to move apart to form a gas. A liquid is being heated, a graph is plotted between time and temperature in °C. Answer the questions based on graph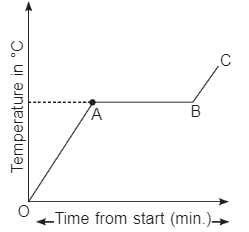 (i) What does AB represent?
(i) What does AB represent?
Ans: It represents a mixture of liquid and vapours at boiling point of liquid.
(ii) What does OA represent?
Ans: It represents liquid is being heated and absorbing energy, therefore, particles move more energetically
(iii) What does BC represent?
Ans: It represents vapour state and particles absorb heat and become more energetic. The tempera-ture of gas will rise
(iv) Why does temperature remain constant at boiling point?
Ans: It is because heat of vapourisation is used to overcome force of attraction between particles of liquid.
Q2: Read the following information and answer the questions based on information and related studied concepts.
Substance – 1. is brittle.
Substance – 2. melts at 5°C and boils at 150°C.
Substance – 3. has high melting point of 800°C.
Substance – 4. has melting point –169°C and boiling point –104°C.
(i) What is physical state of substance – 1 and 3 at room temperature?
Ans: Substance 1 and 3 are solids at room tempera-ture
(ii) Out of substances – 1, 2, 3, 4 which one has strongest force of attraction?
Ans: Substance 3 has strongest forces of attraction.
(iii) What is physical state of substance – 4 at –150°C and –100°C?
Ans: Substance 4 is liquid at –150°C and gas at –100°C
(iv) What is physical state of substance – 2 at 100°?
Ans: It will be in liquid state.
Q3: Read the given passage and answer the questions that follows based on the passage and releted studied concepts.
Matter is anything that occupies space and has mass. Matter is classified into solid, liquid and gas. In solid state particles are closely packed and have very strong force of attraction, particles can only vibrate and rotate around fixed positions. In liquid state, particles are less closely packed and have strong force of attraction but less than solids, particles can move throughout the liquid. In Gaseous state, particles are far apart with weak force of attraction and are in state of constant random motion. Gases can be easily compressed where as solids and liquids are incompressible.
(i) When solid changes into vapours, the process is called.
(a) Evaporation
(b) Boiling
(c) Sublimation
(d) Vapourisation
Ans: (c)
Evaporation is the process by which a liquid changes into vapor or gas at a temperature lower than its boiling point. Sublimation (option c) is the process where a solid directly changes into vapor without passing through the liquid phase, and boiling (option b) refers to the process in which a liquid changes into vapor at its boiling point. Vapourisation (option d) is a more general term that encompasses both evaporation and boiling.
(ii) Why do we feel more cold after taking bath with hot water?
Ans: It is because hot water evaporates faster than cold water and cause more cooling.
(iii) An inflated balloon is placed in refrigerator, what will happen?
(a) Balloon will shrink and particles will move faster and become closer.
(b) Balloon will expand and particles will move faster and become far apart.
(c) Balloon will shrink, particles will move slower and become close together.
(d) Balloon will expand, particles will move slower and come closer therefore, volume of balloon will decrease.
Ans: (c)
Kinetic energy will decrease, so particles will move slower, become closer and volume of balloon will decrease.
(iv) A substance melts at 5°C and boils at 150°C. What will be its physical state at room temperature?
Ans: Liquid

|
Explore Courses for Class 9 exam
|

|

















