Ceramics, Polymers & Composites | Chemistry for Grade 10 PDF Download
Glass and clay ceramics
- Glass ceramics
- Glass is transparent and hard but it is brittle.
 Glass shatters when it is hit or dropped
Glass shatters when it is hit or dropped - Most of the glass we use is soda-lime glass. This is made by melting a mixture of sand (silicon oxide), sodium carbonate and limestone, then allowing the molten liquid to cool and solidify.
- Borosilicate glass is made by heating sand with boron trioxide. Borosilicate glass has a much higher melting point than soda-lime glass.
Example: State and explain which type of glass would be best to make a boiling tube, for use in a school chemistry laboratory.
Borosilicate glass would be best because it has a higher melting point than soda-lime glass. This means that it will not melt when it is heated using a Bunsen burner.
Clay ceramics
- Clay ceramics include brick, china and porcelain. They are made by shaping wet clay and then heating it to a high temperature in a furnace, which causes crystals to form and join together.
- Clay ceramics are often coated with a glaze, which hardens on heating to form a hard, smooth, opaque and waterproof layer. This explains why they are often used for dinner plates and bowls.
 Brick is strong in compression - it resists being squashed
Brick is strong in compression - it resists being squashed
Polymers
Properties of polymers
- Further information on how polymers are made from monomers can be found in More organic chemistry.
- Different polymers have different properties, depending on the monomers they are made from and the conditions under which these monomers were joined together. This means that different polymers have different uses. For example, poly(ethene) can be made in low density and high density forms.
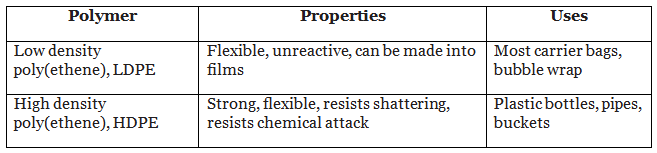
- Low density poly(ethene) has a structure where the polymer chains are branched and this means that the molecules are arranged randomly. High density poly(ethene) has less branching of the polymer chains, so the molecules line up much more closely.
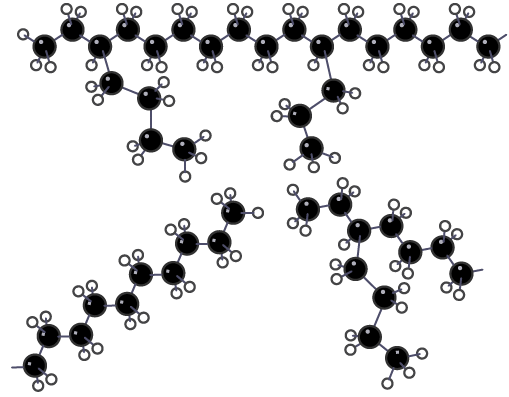 Structure of a low density poly(ethene)
Structure of a low density poly(ethene)
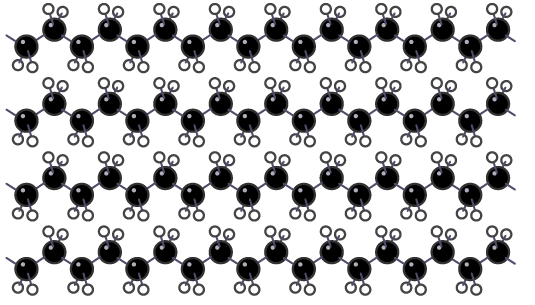 Structure of a high density poly(ethene)
Structure of a high density poly(ethene)
Thermosoftening and thermosetting plastics
Plastics can be put into one of two categories, depending on how they respond when heated.Thermosoftening plastics melt when they are heated. Most plastics that we come across in everyday life are thermosoftening plastics. This means that they can be recycled, which involves melting them before making a new product.
Thermosoftening plastics do not have covalent bonds between neighbouring polymer molecules, so the molecules can move over each other when heated and the plastic melts.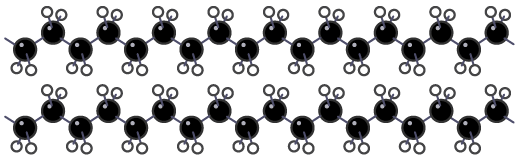 Thermosoftening plastics have no cross links between the polymer molecules
Thermosoftening plastics have no cross links between the polymer molecules
Thermosetting plastics do not melt when heated. They tend to char and burn when heated, but they are resistant to much higher temperatures than thermosoftening plastics. They are used to make electrical plugs, which must not melt, even if there is a malfunction and the wiring inside gets hot.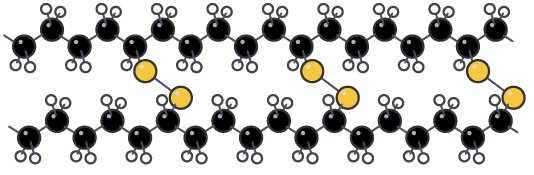 The covalent bonds in this thermosetting plastic are strong and prevent the plastic melting when it is heated
The covalent bonds in this thermosetting plastic are strong and prevent the plastic melting when it is heated
Composite materials
A composite material consists of two or more materials with different properties. They are combined to produce a material with improved properties. Most composite materials have two components:- the reinforcement
- the matrix, which binds the reinforcement together
The table shows some examples of composite materials: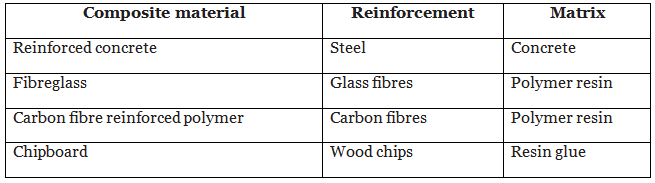
It is often possible to separate the reinforcement from the matrix by physical processes. For example, reinforced concrete can be broken up using machinery. This is one stage in recycling the components of reinforced concrete.
Fibre glass and carbon fibre reinforced polymer (CFRP)
The fibres in these composite materials have a low density. They are strong in tension, so they are not easily stretched, but they are flexible. The polymer resin which binds the fibres together is not strong but it is stiff. The composite materials show a combination of these properties. They are strong, stiff and lightweight.Chipboard
Wood itself is a natural composite material. It consists of a reinforcement of cellulose fibres bonded together by a matrix of lignin. The fibres are aligned alongside each other, so wood is stronger in one direction than it is in the other. Chipboard contains randomly arranged wood chips bonded together by a glue, so it is strong in all directions.
Example: Chipboard may be supplied with a thin polymer layer glued to its surfaces. Suggest reasons that explain why this material is more suitable than chipboard alone for making self-assembly furniture.
The polymer can be coloured or patterned to look like wood. It makes the surface waterproof so it is protected from spills and can be cleaned more easily.
Reinforced concrete
The properties of concrete can be improved by reinforcing it with steel rods or mesh. The compressive strength of concrete is higher than its tensile strength, but the tensile strength of steel is higher than its compressive strength. The combination of the two materials that is strong in tension and in compression. This allows reinforced concrete to be strong and slightly flexible, which is important when constructing large buildings and structures.A comparison of different materials
Different materials have different properties but they may also have some properties in common. The table summarises some of the typical properties of glass and clay ceramics, metals, plastics and composites.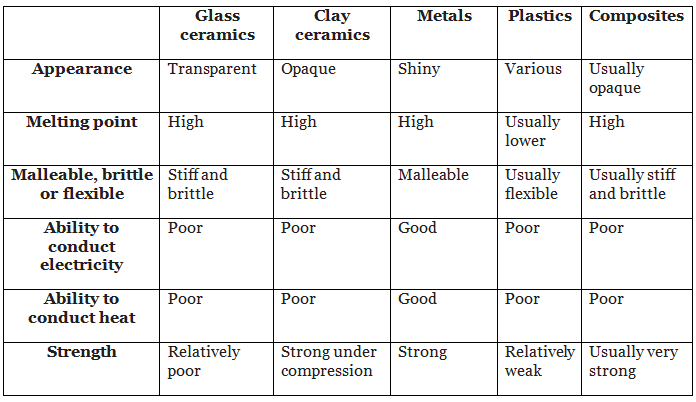
|
75 videos|131 docs|24 tests
|




















