Natural Resources Class 9 Notes Science
| Table of contents |

|
| What are these Resources on the Earth? |

|
| The Breath of Life: Air |

|
| Water: A Wonder Liquid |

|
| Mineral Riches in the Soil |

|
| Biogeochemical Cycles |

|
| Oxygen Cycle |

|
| Ozone Layer |

|
What are these Resources on the Earth?
As per the latest information, life exists only on our planet - The Earth. Life depends upon factors such as air, temperature, water, and food. The resources of the Earth and the energy from the Sun provide the basic requirement of all life forms.
What are Natural Resources?
Air, water, soil, minerals, animals, and plants that are useful to mankind in many ways constitute natural resources.
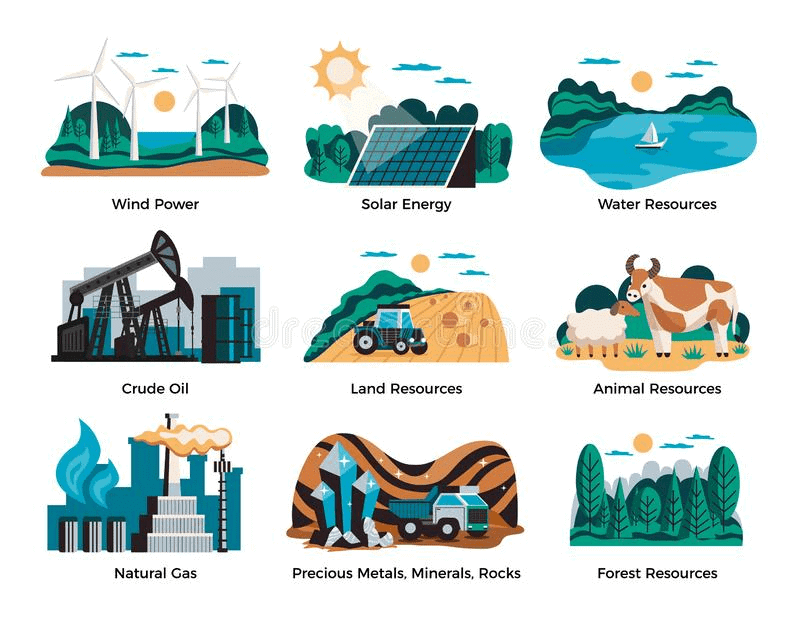 Various Types of Natural Resources
Various Types of Natural Resources
- Atmosphere: The envelope of air that surrounds our planet Earth like a blanket is called the atmosphere.
- Lithosphere: The outer crust of the Earth is known as the lithosphere.
- Hydrosphere: 75% of the Earth’s surface covered with water, comprising seas, oceans, rivers, lakes, ponds, etc., is called hydrosphere. Underground water is also covered under the hydrosphere.
- Biosphere: Life supporting zone of the Earth where the atmosphere, the hydrosphere, and the lithosphere interact and make life possible, is known as the biosphere.
(i) Biotic components: Living things like plants, animals, microorganisms, etc., of the biosphere constitute the biotic components.
(ii) Abiotic components: The air, the water, and soil of the biosphere form the abiotic or non-living components.
The Breath of Life: Air
Composition of Air
- It is a mixture of many gases like nitrogen, oxygen, carbon dioxide and water vapour.
- Nearly 78% and 21% of air is nitrogen and oxygen respectively.
- Carbon dioxide is present in small amounts.
- Noble gases like helium, neon, argon and krypton are present in traces.
- It is amazing to note that carbon dioxide constitutes up to 95-97% in the atmospheres of Venus and Mars. That is why no life is possible there.
The Role of the Atmosphere in Climate Control
- Air is a bad conductor of heat.
- The atmosphere keeps the average temperature of the Earth fairly stable during the day and during the year. It prevents a sudden increase in temperature during the day.
- During the night, it slows down the escape of heat into outer space preventing nights from becoming terribly cold.
- Compare with the situation on the moon where there is no atmosphere and the temperature varies from — 190 °C to 110 °C
The Movement of Air - Winds
- Cool breeze after a hot day and rains after days of hot weather are the result of changes that take place in our atmosphere due to the heating of air and the formation of water vapour when air is heated by radiation from the heated land or water, it rises.
- Since land gets heated faster than water, air over the land also gets heated faster than air over water bodies.
- Consider yourself in a coastal area during the day, the air above the land gets heated faster and starts rising. As air rises a region of low pressure is created and air over the sea moves in to this area of low pressure. The movement of air from one region to the other creates winds.
- At night, both land and sea start to cool. Since water cools down slower than the land, the air above water would be warmer than the air above land. Thus, the movement of air will take place from land to sea.
- The movement of air is also caused by uneven heating of the atmosphere in different regions of the Earth. The rotation of Earth and the presence of mountain ranges in the paths of wind are other factors that affect the wind.
Rain
- When water bodies are heated during the day, a large amount of water evaporates and goes into the air. Some amount of water vapours also get into the atmosphere because of various biological activities.
- The hot air rises up carrying the water vapours with it. As the air rises, it expands and cools. The cooling causes the water vapour in the air to condense in the form of tiny droplets.
- The condensation of water is facilitated if some particles could act as the ‘nucleus’ for these drops to form around. Dust and other suspended particles perform this function when the drops have grown big and heavy, they fall down in the form of rain.
- When the temperature of the air is low, precipitation may occur in the form of snow or hail.
Air Pollution
- Fossil fuels like coal and petroleum contain small amounts of nitrogen and sulphur. When these are burnt, nitrogen and sulphur are also burnt to produce oxides of nitrogen and sulphur.

- Inhalation of these gases is dangerous. Also, these gases dissolve in rainwater to cause acid rain.
- The burning of fossil fuel also increases the number of suspended particles, in the form of unburnt carbon particles or hydrocarbons, in the air.
- A high level of pollutants in the atmosphere lowers visibility, especially in cold weather. Smog (smoke + fog) is the result of this kind of pollution.
- Breathing polluted air increases the incidence of respiratory problems, allergies, cancer and heart diseases.
Water: A Wonder Liquid
- A wonder Liquid. About 75% of the Earth surface is covered with water. Water is also found underground.
- Most of the water on Earth’s surface is found in seas and oceans and is saline. Freshwater is found frozen in the ice caps at the two poles and on snow-covered mountains.
- The underground water and water in rivers, lakes and ponds are also fresh.
- In spite of the huge presence of water on the Earth’s surface, there is always a shortage of drinking water practically everywhere.
Necessity of Water
- All cellular processes take place in the water medium.
- All the reactions that take place within our body and within the cells occur between substances that are dissolved in water. Substances are also transported from one part of the body to the other in a dissolved form.
- Terrestrial life-forms require freshwater because their bodies cannot tolerate the high amounts of dissolved salts in saline water.
- The availability of water decides not only the number of individuals of each species that are able to survive in a particular area, but it also decides the diversity of life there.
- Water is one of the major resources which determine life on land.
Water Pollution
- Water dissolves the fertilisers and pesticides that we use in agriculture. Some fraction of these substances are washed into water-bodies. Sewage from the towns and cities and the industrial wastes are also dumped into rivers and lakes.
 Discharge of Industrial Waste
Discharge of Industrial Waste
- The temperature of water in the water-bodies is affected when water is released from the dams.
Explanation: Water inside the deep reservoir would be colder than water at the surface which gets heated by the Sun. This can affect life forms that are found in these water bodies in various ways. It can encourage the growth of some life forms while it damages other life forms. This affects the balance between various organisms which had been established in that system.
Harmful Effects of Water Pollution
- Certain substances like fertilisers and insecticides which are used in farming are poisonous substances. These are carried by water to the water bodies. Also, some disease-causing bacteria are carried by water to water bodies.
- Dissolved oxygen is used by plants and animals that live in water. Industrial and other wastes reduce the oxygen content in water thereby making it difficult for aquatic life to survive.
- Aquatic organisms are used to a certain range of temperature in the water bodies where they live. Sudden change in the temperature as a result of water pollution would be dangerous for them or affect their breeding.
Mineral Riches in the Soil
- The upper layer of the Earth is called the soil. Minerals found in this layer supply a variety of nutrients to life forms.
- Over a long period of time, thousands and millions of years, the rocks at or near the surface of Earth are broken down by various physical, chemical and some biological processes. The end product of this breaking down is the fine particles of soil.
Processes That Make Soil
- Sun: The Sun heats up the rocks during the day so that they expand. They contract when the rocks cool down at night, this leads to cracks in the rocks. Ultimately, rocks change into smaller pieces.
- Water: Water gets into the cracks in the rocks. If this water freezes later, it could cause wider cracks. Then flowing water wears away even hard rock over a period of time. Flowing water carries big and small particles of rock downstream. Soil is thus found in places far away from its parent rocks.
- Wind: Strong winds also rub against rocks and wears them down. The wind carries the sand from one place to the other.
- Living Organism: Lichens grow on the surface of rocks. They release certain substances that cause the rock surface to powder down and form a thin layer of soil. Moss grows on this soil and causes the rocks to break up further.
Constituents of Soil
- Soil contains small particles of rock, bits of decayed living organisms called humus and various other forms of microscopic life.
- The type of the soil is decided by the average size of particles found in it while the quality of the soil is decided by the amount of humus and microscopic organisms found in it.
- Humus causes the soil to become more porous and allows water and air to penetrate deep underground.
- The nutrient content of a soil, the amount of humus present in it and the depth of the soil are some of the factors that decide which plants will grow on that soil.
Soil Pollution
- Large quantities of fertiliser and insecticides, which are used in modern farming, can destroy the soil structure by killing the micro-organisms that recycle nutrients in the soil.
- It also kills the earthworm which is instrumental in making rich humus.
- Removal of useful components from the soil and the addition of other substances, which adversely affect the fertility of the soil and kill the diversity of organisms that live in it, is called soil pollution.
Soil Erosion
- The fine particles of topsoil may be carried away by flowing water or wind. It is called soil erosion.
- If all the soil gets washed away and the rocks underneath are exposed, we have lost a valuable resource because very little will grow on the rock.
- Activity: Perform an activity to show that flowing water can cause soil erosion.
Materials required: Two trays, soil, mustard seeds.
Procedure:
(i) Take two identical trays and fill them with soil.
(ii) Plant mustard seeds in one of them and water both the trays regularly for a few days till one tray is covered with mustard growth.
(iii) Tilt both the trays and fix them in that position. Make sure that both trays are inclined at the same angle.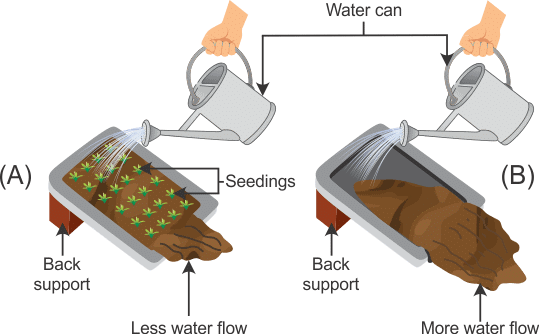 (iv) Pour equal amounts of water gently on both; the trays such that water flows out of the trays.
(iv) Pour equal amounts of water gently on both; the trays such that water flows out of the trays.
(v) Study the amount of soil that is carried out of the trays.
(vi) Now pour equal amounts of water on both the trays from a height. Again study the amount of soil that is carried out of the trays.
Observation: It is observed that soil carried out in the tray with mustard growth is much less than that carried out in the other tray, both when water is dropped gently and from a height. This is so because the roots of plants bind the soil particles together thereby preventing soil erosion.
Prevention of Soil Erosion
- One of the causes of soil erosion is mass-scale deforestation that is happening all over the world. It not only destroys biodiversity but also causes soil erosion.
- The consequence of soil erosion is that the topsoil which has no vegetation on it is likely to be removed very soon. The situation is all the more serious in hilly or mountainous regions. Vegetative cover on the soil plays a vital role in the percolation of water into deeper layers and helps in raising the water table also.
Biogeochemical Cycles
There is a constant interaction between biotic and abiotic components of a biosphere and it is a dynamic and stable system. There is a transfer of matter and energy between the different components of the biosphere.
Water Cycle
- The whole process in which water evaporates and falls on the land as rain and later flows back into the sea via rivers is known as the water cycle.
- All of the water that falls on the land does not immediately flow back into the sea. Some of it seeps into the soil and becomes part of the underground reservoir of freshwater.
Some of this underground water finds its way to the surface through springs or this is brought out to the surface for our use through wells or tube wells. - Water is used by terrestrial animals and plants for various life processes.
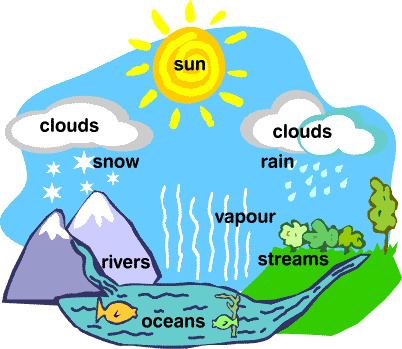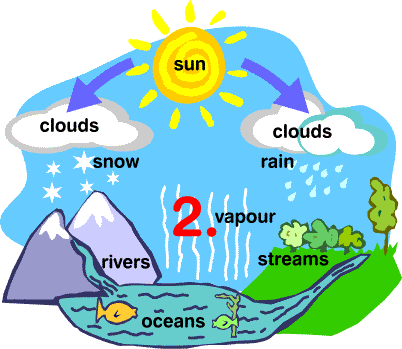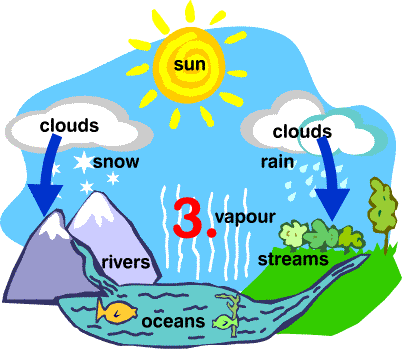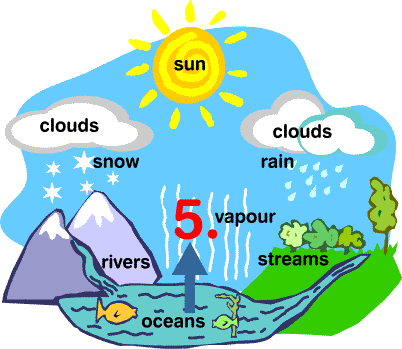
- As water flows through rocks containing soluble minerals, some of them get dissolved in water. Thus, rivers carry many nutrients from the land to the sea, and these are used by marine organisms.
Nitrogen Cycle
- Nitrogen gas makes up 78% of our atmosphere. Nitrogen is an essential nutrient for all life forms.
- However, except for a few forms of bacteria, life-forms are not able to convert inert nitrogen from the air into nitrates and nitrites which could be taken up and used to make molecules like proteins, nucleic acids and vitamins etc.
- The nitrogen-fixing bacteria are found in the roots of legumes in special structures called root nodules.
- Another way nitrogen can be converted into nitrites and nitrates is by lightning when nitrogen and oxygen in the atmosphere under the influence of high temperature and pressure are converted into oxides of nitrogen. These oxides dissolve in water to give nitrous and nitric acids and fall on land along with rain.
- The whole process in which elemental nitrogen of the atmosphere passes into the soil and water in the form of simple molecules which get converted to more complex molecules in a living being and go back to the atmosphere as simple nitrogen molecules are called the nitrogen cycle.
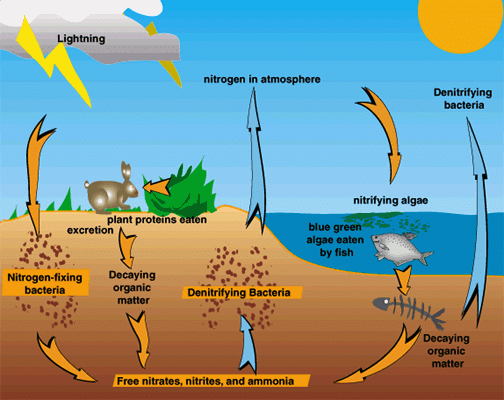
Processes involved in the Nitrogen Cycle:
(i) Ammonification: It is the process of decomposition of dead organic matter into ammonia. This is done by micro-organisms living in the soil e.g., decay bacteria and fungi.
(ii) Nitrification: It is the process of conversion of ammonia into nitrites and then into nitrates. This is done by nitrifying bacteria Nitrosomonas and Nitrobacter.
(iii) Denitrification: It is the process of reducing nitrates present in the soil to release nitrogen gas back into the atmosphere with the help of the bacteria Pseudomonas.
Carbon Cycle
- All life forms are based on carbon-containing molecules like proteins, carbohydrates, fats, etc.
- The endoskeletons and exoskeletons of various animals are also formed from carbonate salts.
- Carbon gets included into the life-forms through the basic process of photosynthesis that takes place in the presence of sunlight by all life form that contains chlorophyll.
- In this process, the carbon dioxide from the atmosphere or dissolved in water gets converted into glucose molecules. These glucose molecules are used to provide energy for the synthesis of other biologically important molecules.
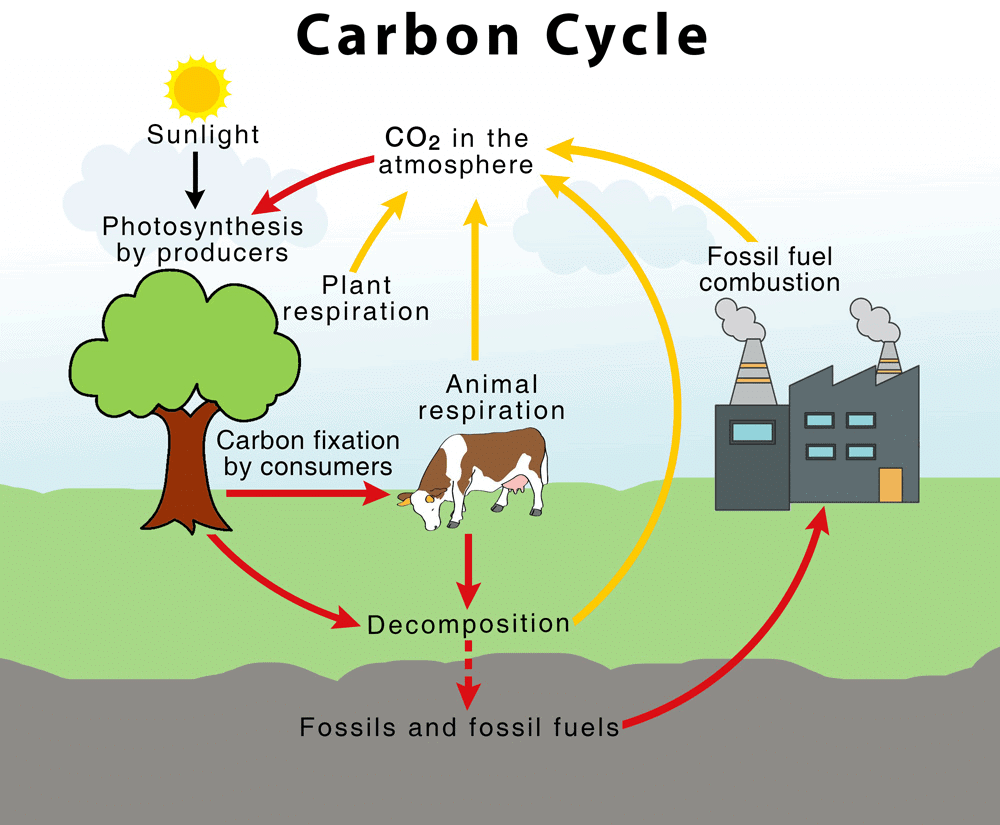
- During respiration, glucose is oxidised to carbon dioxide again which goes to the atmosphere.
- Carbon dioxide is also added to the atmosphere by the combustion of fossil fuels.
- Carbon is thus cycled repeatedly through different forms by various physical and biological activities.
Greenhouse Effect
- It is an effect occurring in the atmosphere because of the presence of certain gases like carbon dioxide that absorb infrared (heat) radiations, thereby increasing the temperature worldwide.
- A greenhouse is a structure to protect the plants from the cold atmosphere. The plants are kept in an enclosure. Heat rays from the Sun enter the greenhouse and are trapped there.
- Heat does not escape easily keeping the temperature warm inside.
- The carbon dioxide layer around the earth performs the same function. It traps the heat of the Sun and then does not allow it to escape. This results in raising the average temperature, that is, it results in warming of the atmosphere.
- Global warming that is taking place these days is the result of the greenhouse effect. There is a need to check the level of carbon dioxide in the atmosphere.
Oxygen Cycle
- Oxygen is present in the elemental form in the atmosphere to the extent of 21%. It is also present in Earth’s crust and as carbon dioxide in the atmosphere.
- It is present in the crust in the form of oxides of metals and as carbonate, sulphate and nitrate.
- It is an essential component of carbohydrates, protein, fats and nucleic acids.
- It is present in dissolved form in water bodies and helps in the survival of aquatic animals.
- The oxygen cycle maintains the level of oxygen in the atmosphere. Oxygen from the atmosphere is used up in three processes namely combustion, respiration and the formation of oxides of nitrogen. It is vital for all living organisms for respiration.
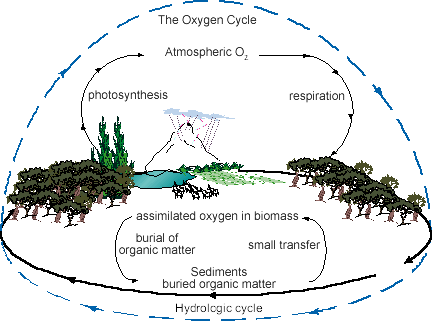 The Oxygen Cycle
The Oxygen Cycle- Oxygen is returned to the atmosphere by one major process i.e., photosynthesis and this forms the basis of the oxygen cycle in nature.
Ozone Layer
- Normally elemental oxygen is found in the diatomic form. But, in the upper reaches of the atmosphere, a molecule containing three atoms of oxygen is formed. This is called ozone.
- It is a poisonous substance but it performs an essential function where it is found. It absorbs harmful ultra-violet radiations from the Sun. This prevents those radiations from reaching the surface of the Earth where they may damage many forms of life.
- Recently it was discovered that this ozone layer was getting depleted.
- Various manmade compounds like CFCs (chlorofluorocarbons) which are not degraded by any biological process, reach the ozone layer and react; with ozone molecules. This has caused the depletion of the ozone layer over Antarctica.
- If the ozone layer dwindles further, the consequences will be disastrous.
|
19 videos|94 docs|52 tests
|
FAQs on Natural Resources Class 9 Notes Science
| 1. What is the role of air in sustaining life on Earth? |  |
| 2. Why is water considered a wonder liquid? |  |
| 3. What are mineral riches in the soil, and why are they important? |  |
| 4. How do biogeochemical cycles contribute to the Earth's ecosystem? |  |
| 5. What is the ozone layer, and why is it important for life on Earth? |  |





















