Chemical Cells & Fuel Cells | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Chemical cells |

|
| Fuel cells |

|
| Evaluating different cells |

|
| Fuel cells in spacecraft |

|
Chemical cells
Chemical cells use chemical reactions to transfer energy by electricity. The voltage of a cell depends upon a number of factors, including what the electrodes are made from, and the substance used as the electrolyte.
- A simple cell can be made by connecting two different metals in contact with an electrolyte. A number of cells can be connected in series to make a battery, which has a higher voltage than a single cell.
- In non-rechargeable cells, eg alkaline cells, a voltage is produced until one of the reactants is used up. When this happens, we say the battery ‘goes flat’.
- In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an external circuit is supplied.
What affects the voltage of a cell?
Here is a simple reactivity series: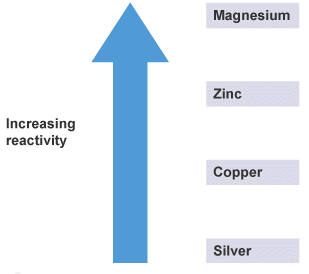
A simple reactivity series
If we connect different combinations of these metals to make a cell, we find that the voltage changes. In the below table, the positive electrodes and what they are made from are listed along the top and the negative electrodes along the side.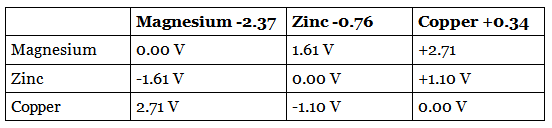
Swapping the two electrodes means that the recorded voltage becomes negative. The biggest voltage occurs when the difference in the reactivity of the two metals is the largest. A cell made from magnesium and copper has a higher voltage than either of the other two combinations.
Example: Suggest the voltage of a cell made using magnesium and silver
Any value greater than 2.71 V would be an acceptable answer. This is because the difference between the reactivity of magnesium and silver is greater than the difference between magnesium and copper. In fact, the cell would have a voltage of approximately 3.5 V.
Fuel cells
Fuel cells work in a different way than chemical cells. Fuel cells produce a voltage continuously, as long as they are supplied with:- a constant supply of a suitable fuel
- oxygen, eg from the air
The fuel is oxidised electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Energy is released as electrical energy, not thermal energy (heat).
Hydrogen-oxygen fuel cells
hydrogen + oxygen → water
2H2(g) + O2(g) → 2H2O(l)
Electrode half equations - Higher
At the negative electrode: 2H2 + 4OH- → 4H2O + 4e-
At the positive electrode: O2 + 2H2O + 4e- → 4OH-
When you add these two half equations together, you get the following overall equation:
2H2 + 4OH- + O2 + 2H2O + 4e- → 4H2O + 4e- + 4OH-
The hydroxide ions, electrons and two H2O molecules will now cancel because they are on both sides, leaving the overall equation:
2H2 + O2 → 2H2O
Evaluating different cells

Fuel cells have different strengths and weaknesses, depending on the intended use. For example, fuel cells are used in spacecraft and vehicles.
Fuel cells in spacecraft
- Hydrogen-oxygen fuel cells are used in spacecraft. In addition to the strengths in the table above, the water they produce is useful as drinking water for astronauts.
- Hydrogen-oxygen fuel cells must be supplied with hydrogen fuel and oxygen. This could be a problem once a spacecraft leaves the Earth. However, spacecraft in orbit, such as the International Space Station, have solar cells. These convert light into electricity, so the hydrogen and oxygen can be replaced by the electrolysis of water.
- Solar cells only work when they are in the light, so the fuel cells allow electricity to be produced even when the spacecraft is in the dark.
|
78 videos|87 docs|11 tests
|

















