Class 9 Science: Sample Question Paper- 7 (With Solutions) PDF Download
(Section - A)
Q.1. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show ?
Ans. Particles of water are held together by forces of attraction. It is these forces of attraction, which the diver cuts through water in the swimming pool.
Q.2. The property to flow is unique to fluids. Which one of the following statements is correct?
(a) Only gases behave like fluids
(b) Gases and solids behave like fluids
(c) Gases and liquids behave like fluids
(d) Only liquids are fluids
Ans. (c)
Liquids and gases are called fluids because of their ability to flow. The fluidity in both of these states as the molecules are free to move due to lesser force of attraction between the particles. On the contrary; the constituent particles in solids have fixed positions and can only oscillate about their mean positions so does not flow.
Q.3. A man raises a weight of 1 kg to a height of 1 m and holds it there for 30 s. How much work has he performed ?
(a) 9.8 J
(b) 9.8 x 30 J
(c) 9.8 x 31 J
(d) zero
Ans. (a) Here m = 1 kg and h = 1 m
∴ Work done W = F s = (mg).h = 1 x 9.8 x 1 = 9.8 J
Q.4. Question number 4 is based on the paragraph given below. Study this paragraph and answer the questions that follow.
When two or more non-reacting gases are kept side by side, they have the tendency to mix with one another to form a homogeneous mixture. This can occur also if two gases have different densities. The heavier gas can move up or a lighter gas can move down against the action of gravity. It shows that gas particles move at random with large intermolecular spaces.
This mixing of gases is known as diffusion. According to Graham's law of diffusion, the rate of diffusion of a gas is inversely proportional to the square root of its density or molar mass. Thus, gas having lower molecular mass will diffuse fast
(i). Which of the following gases will have the highest rate of diffusion ? ...
(a) O2
(h) CO2
(c) NH3
(d) N2
Ans. (c)
(ii). Non-reacting gases have a tendency to mix with each other. This phenomenon is known as :
(a) effusion
(b) diffusion
(c) chemical reaction
(d) explosion.
Ans. (b)
(iii). The rate of diffusion of hydrogen is about:
(a) only half of that of helium
(b) 1.4 times that of helium
(c) twice that of helium
(d) four times that of helium
Ans. (b)
(iv). Rate of diffusion of a gas is :
(a) directly proportional to its molecular mass
(b) inversely propositional to its molecular mass
(c) inversely proportional to the square root of its molecular mass
(d) directly proportional to the square root of its molecular mass
Ans. (c)
Q.5. Which of the following correctly represent the electronic distribution in the Mg atom?
(a) 3, 8, 1
(b) 2, 8, 2
(c) 1, 8, 3
(d) 8, 2, 2
Ans. (b)
Atomic number and the number of electrons in magnesium atom is 12. So, electronic configuration is 2, 8, 2.
Q.6. Which of the following solutions show Tyndall effect ?
(a) Solution of copper sulphate
(b) Solution of common salt
(c) Muddy water
(d) Starch solution
Ans. (c) and (d)
Q.7. The bells of a temple are made of large size. It is for :
(a) producing sound of high pitch
(b) producing loud sound
(c) producing sound of high quality
(d) enhancing the beauty
Ans. (b)
Q.8. Choose the wrong statement.
(a) The nature of matrix differs according to the function of the tissue.
(b) Fats are stored below the skin and in between the internal organs
(c) Epithelial tissues have intercellular spaces between them.
(d) Cells of striated muscles are multinucleate and unbranched.
OR
Parenchyma ceils are
(a) relatively unspecified and thin walled.
(b) thick walled and specialized,
(c) lignified.
(d) none of the above.
Ans. (a)
The nature of matrix differs according to their function. For e.g., cartilage has calcium salts as it provides support to bones whereas muscles possess contractile proteins for their function of movement
OR
(a)
The layers of parenchyma cells form the basic packing tissue of plants The cells of this tissue are live, thin walled and relatively unspecialised.
Q.9. Identify the incorrect statement :
(а) In aves forelimbs are modified as wings and they breathe through lungs.
(b) Aves are warm blooded and have four chambered heart.
(c) Platypus is an egg laying mammal.
(d) Crocodile is a reptile having three chambered heart.
Ans. (d) Aves, Mammals and Crocodile have a four chambered heart while other reptile have a three chambered heart.
Q.10. Survival of plants in terrestrial environment has been made possible by the presence of :
(a) Intercalary meristem
(b) Conducting tissue
(c) Apical meristem
(d) Parenchymatous tissue
Ans. (b)
Q.11. Write the formula to calculate the speed of a body moving along a circular path of radius 'r' when it has completed n revolutions in time t.
OR
Mention the physical quantity shown by the slope of a speed -time graph.
Ans.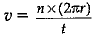
Or
Acceleration
Q.12. Two bones are connected to each other with the help of _____________.
Ans. ligament
Q.13. For question numbers 13 and 14 two statements are given-one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (i), (ii), (iii) and (iv) as given below.
(i) Both A and R are true and R is correct explanation of the assertion.
(ii) Both A and R are true but R is not the correct explanation of the assertion.
(iii) A is true but R is false.
(iv) A is false but R is true.
Assertion: The covering or protective tissues in the animal body are epithelial tissues.
(i) Epithelium covers most organs and cavities within the body.
(ii) It also form a barrier to keep different body systems separate.
Reason: The skin, the lining of the mouth, the lining of blood vessels, lung alveoli and kidney tubules are all made of epithelial tissue. Epithelial tissue cells are tightly packed and form a continuous sheet. They have only a small amount of cementing material between them and almost no intercellular spaces.
(i) A
(ii) B
(iii) C
(iv) D
Ans. (i)
Q.14. The molecules of water have more energy as compared to molecules of ice at same temperature. Justify this statement.
OR
When 2 ml of dettol is dissolved in 100 ml of water, the smell can be detected even on repeated dilution. Identify the physical nature of matter.
Ans. Water has more energy than ice at same temperature because particles in water have absorbed more energy during the change of state.
(Section - B)
Q.15. A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Ans.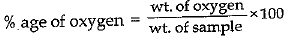
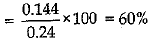
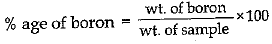

Q.16. Distinguish between longitudinal and transverse waves with one example each.
Ans. Differences between longitudinal and transverse waves :
Q.17. What do you mean by the term ‘pitch of a sound ?
Draw a diagram depicting low pitched sound and high pitched sound. What is the main difference between the two ?
Or
Establish a relation between wavelength, frequency and speed of sound in a medium.
Ans. Pitch is that characteristic of sound which enables us to distinguish between a shrill sound (sound of higher pitch) from a grave or a hoarse sound (sound of lower pitch).
The diagrams have been shown in the following figure.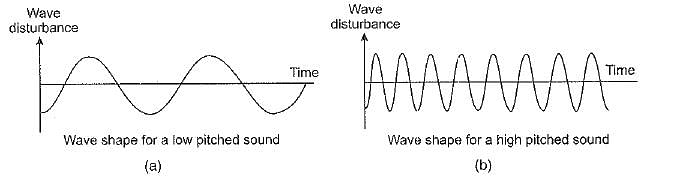
High pitched sound has high frequency but low pitched sound has low frequency.
Or
The speed of sound is defined as the distance which a point on a wave travels in a unit time.
As distance covered by wave in one time period is called the wavelength, hence
But 1/T = number of vibrations completed per unit time = v = frequency
∴ 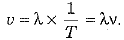
or Speed of sound (v) = frequency (v) x wavelength (λ)
Q.18. (a) Label a, b, c and d in given fig.
Give the function of (b).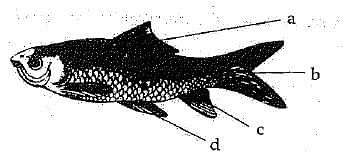
(b) To which class ofan im a lia does the housefly belong ? Mention any three characteristic features of this class of animals. Give two other examples of this class of animals.
Or
(a) You have seen weather reports on television and in newspapers. How do you think we are able to predict the weather?
(b) Describe how lichens and big trees influence the formation of soil
Ans. (a)
a → Dorsal fin
b → Caudal fin
c → Pelvic fin
d → Pectoral fin
Function of Caudal fin- Caudal fin helps in streamlined movement in water.
(b) Arthropoda
Characteristic features:
bilaterally symmetrical and segmented
open circulatory system
blood filled coelomic cavity
have jointed legs
Examples : Butterfly, scorpion, cockroach, centipede, spider, prawn.
Or
(a) Weather observatories collect information regarding the pattern of temperature, speed of wind, air pressure, ocean features and all other features which can affect the weather. This information is collected by remote sensing and weather forecasting satellites. The information collected is then sent to the meteorological departments which prepare a weather report which is displayed on the maps. This information is further transmitted through radio and television.
You might have heard about weather report saying Jdepressions' in the Way of Bengal have caused rains in some areas.
(b)
(i) Lichens grow on the surface of rocks and release substances that powder down the rock surface.
(ii) Moss grows on this surface and breaks it further.
(iii) The roots of trees grow into rocks, form cracks and widen them further to form soil.
Q.19. (a) What is the function of notochord ?
(b) List out any four features that all chordates will possess.
Ans.
(a) Provides spate for muscles io attach for case of movement.
(b) Chordates:
(i) have a notochord
(ii) have a dorsal nerve cord
(iii) arc triploblastic
(iv) have paired gill pouches
(v) are coelomate
Q.20. Suggest a scheme for the separation of constituents of the mixture of sulphur + sand + sugar + iron filings.
Or
Suggest a scheme for the separation of constituents of the following mixture : ammonium chloride + sand + sodium chloride.
Ans. First of all, iron filings are separated by moving a horse shoe magnet over the mixture repeatedly. The remaining mixture is then treated with water to dissolve sugar which is recovered by evaporation of aqueous solution.
Insoluble sulphur and sand are treated with carbon disulphide which dissolves sulphur leaving behind sand. Sulphur is obtained by evaporation of CS2.
Or
Scheme of separation for ammonium chloride + sand + sodium chloride :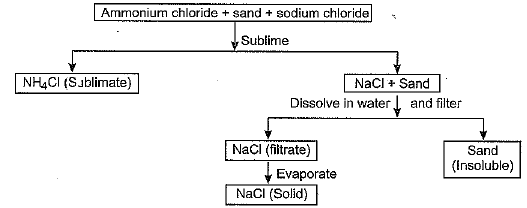
Q.21. (a) Name the organelle which provides turgidity and rigidity to the plant cell. Name any two substances which are present in it.
(b) How are they useful in unicellular organisms ?
Ans. (a) Vacuoles;
Amino acids, proteins, various carbonic acids, sugars.
(b) (i) In amoeba, food vacuole contains the food items.
(ii) In some other unicellular organism, specialised vacuole plays important role in expelling excess water and some waste from the cell.
Q.22. (i) State Newton's third law of motion.
(ii) If someone jumps to the shore from a boat, the boat moves in the opposite direction. Explain.
(iii) When air from an inflated balloon is allowed to be released, the balloon moves in a direction opposite to that of air. Explain.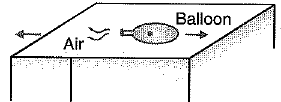
Ans. (i) To every action, there is always an equal and opposite reaction.
(ii) The boat moves in the opposite direction due to the force exerted by the man to help him move in the forward direction which is the reaction force.
(iii) The rushing of air out of the balloon due to the pressure difference exerts ahequal and opposite force on the balloon which causes the latter to move in the opposite direction Of the released air.
Q.23. (i) How is smog formed ?
(ii) What is ‘organic farming’ ?
Or
Explain the basis of Principle of Treatment for any disease.
Ans. (i) Smog is formed by combustion of fossil fuels which increases the amount of suspended particles of unburnt carbon or hydrocarbons. Their presence lowers the visibility, specially in cold weather when water also condenses in the air.
(ii) Organic farming is a system of farming without the use of chemicals such as fertilisers and pesticides etc., and with a maximum input of organic manures with healthy cropping systems.
Or
There are two ways to treat an infectious disease. They are as follows :
(i) Reduce the effect of disease : The principle involved is that the effect of disease is lessened without killing the infectious agent which is done either by taking medicines and antibiotics as well as taking appropriate rest so that the body heals.
(ii) Kill the microorganisms of infectious agents : The infectious agents like bacteria, viruses, fungi, helminths and protozoan’s have some essential biochemical life processes which are peculiar to this group. These processes may be pathways for respiration or synthesis of new substances. Drugs are available which block these processes and kill the infectious agents. Antibiotics are chemicals produced by microbes which kill or prevent the growth of other microbe by blocking life processes without harming human cells.
Q.24. Using following data draw time - displacement graph for a moving object: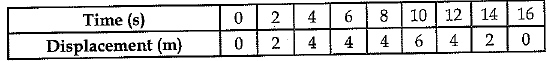
Use this graph to find average velocity for first 4 s, for next 4 s and for last 6 s.
Or
A ball is gently dropped fro m a height of 20 m. If its velocity increases uniformly at the rate of 10 ms-2 with what velocity will it strike the ground? After what time will it strike the ground?
Ans. The graph is as shown: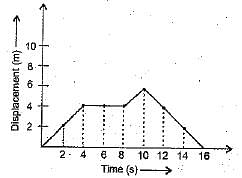
Average velocity for first 4 s = slope of the part OA of the graph = 4/4 = 1 m s-1
Average velocity for next 4 s = 0 (graph is a straight line)
Average velocity for the last 6 s = (0 - 6)/ (16 - 10) = - 1 m s-1
Or
Given: Initial velocity of ball u = 0
Final velocity of ball, v =?
Distance through which the ball falls, S = 20 m
Acceleration, a = 10 m s-2
Time of fall, t = ?
We know
v2 - u2 = 2aS
or v2 - 0 = 2 x 10 x 20 = 400
or v = 20 m s-1
Now, using v = u + at, we have
20 = 0 + 10 x t
or t = 2 s
(Section - C)
Q.25. (a) Compare metals and non-metals based on their physical properties, (any four points)
(b) What are metalloids ? Give two examples.
(c) Identify metals from the following : Boron, sodium, mercury, carbon.
Or
(a) A sample of vitamin C is known to contain 2.58 x 1024 oxygen atoms. How many moles of oxygen atoms are present in the sample ?
(b) Write one word for the following:
(i) In a balanced chemical equation, the sum of the masses of reactant and products remains unchanged.
(ii) A group of atoms carrying a fixed charge on them.
(c) Write chemical formulae of the following compound:
(i) Sodium phosphate
(ii) Ammonium carbonate
Ans.

(b) The elements with intermediate properties between those of metals and non-metals are called metalloids, e.g., Boron, silicon, germanium, etc.
(c) Sodium and mercury are metals
Or
(a) 6.022 x 1023 oxygen atoms are present in 1 mole oxygen atom
2.58 x 1024 oxygen atoms are present in 
(b) (i) Law of conservation of mass.
(ii) Polyatomic ion.
(c) (i) Na3PO4
(ii) (NH4)2CO3
Q.26. (i) Prove that if the earth attracts two bodies placed at the same distance from the centre of earth, with equal force; then their masses will be the same. (ii) Mathematically express the acceleration due to gravity that is expressed by a free falling object,
(iii) Why is 'G' called a universal constant ?
Ans. (i) Let mass of first body be m1
Let mass of second body be m2
Force on 1st body = Force on 2nd body
GMm1/R2 = GMm2/R2
G and G cancel. M and M cancel R2 and R2 cancel
This leaves
m1 = m2
Hence proved.
(ii) g. = GM/R2
(iii) Its value is constant in the universe.
Q.27. (a) How many electrons, protons and neutrons will be there in an element 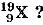 What will be the valency of this element ?
What will be the valency of this element ?
(b) What are isotopes ? Write the three isotopes of hydrogen atom. Write any two applications of an isotope.
Ans. (a) Mass number of X = 19
Atomic number of X = 9
Therefore number of protons = Number of electrons = 9
Number of neutrons = Mass number - Atomic number
= 19 - 9 = 10
The electronic distribution in X is 2, 7.
It will gain one electron to complete its octet.
Therefore, valency of X = 1
(b) Isotopes are defined as the atoms of the same element having the same atomic number but different mass numbers.
Q.28. "There is a need for sustainable practices in agriculture and animal husbandry". Mention the reason for their need.
Or
(a) What do the blanks 1, 2, 3,4 and 5 in the given cycle stand for ?
(b) Name two natural and one man-made process by which CO2 returns to the atmosphere.
(c) Carbon dioxide is necessary for plants but it is also a pollutant. Justify your answer.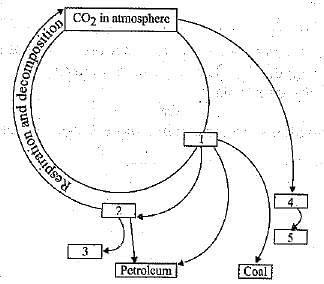
Ans. (i) There is an increase in foodgrain production because of green revolution. Similarly, white revolution has led to better and more efficient use as well as availability of milk.
(ii) These revolutions have led to intensive utilisation of natural resources and now there are more chances of causing damage to our natural resources to the point of destroying their balance completely.
(iii) Therefore, it is important that we should increase food production without degrading our environment and disturbing the balances that maintains it. Hence, it is said that, there is a need for sustainable practices in agriculture and animal husbandary.
Or
(a) 1 - Organic compounds (plants)
2 - Organic compounds (animals)
3 - Inorganic carbonates (shells)
4 - Carbonates in water
5 - Limestone
(b) Respiration.
Decomposition.
Combustion of fuel.
(c) (i) CO2 causes the average temperature to increase.
(ii) CO2 is responsible for global warming
Q.29. Describe Rutherford's α-partide scattering experiment and mention the important observations and conclusions drawn from this experiment.
Ans. In this experiment fast moving α-particles were made to fall on a thin gold foil.
The following observations were made :
(i) Most of the fast moving α-particles passed straight through the gold foil.
(ii) Some of the α-particles were deflected by the foil by small angles.
(iii) One out of every 12000 particles appeared to rebound.
Conclusion:
(i) Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
(ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of α-particles was deflected by 180º, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
Q.30. Planaria and Ascaris are triploblastic. Will you classify them in one group ? If no, name the groups and two important characteristics based on which you will characterise them in their respective groups.
Ans. No.
Planaria - Platyhelminthes
Ascaris - Nematoda/Nemathelminthes
Characteristics of Phylum Platyhelminthes :
(i) A phylum of flatworms where the body is dorsoventrally flattened, triploblastic but acoelomate with organ level of organisation and bilateral symmetry.
(ii) They possess soft, elongated leaf like or tape like body which exhibits bilateral symmetry.
(iii) Platyhelminthes are mostly parasitic, some are free living {Planaria),
(iv) Respiratory and circulatory systems are underdeveloped.
(u) Nervous system is well developed. Primitive brain is also formed.
(vi) They are bisexual or hermaphrodites.
Characteristics of Phylum Nematoda or Nemathelminthes :
(i) Nematoda is a phylum of triploblastic, bilaterally symmetrical but cylindrical worms having pseudocoelom, primitive organ system level of organisation and an elastic cuticle on the outside.
(ii) Some round worms are free living in soil or water. Most of the others are parasitic (filarial worm).
(iii) Parasitic roundworm like filarial worms cause a disease known as elephantiasis in which the leg of the patient becomes swollen (just like leg of elephant).
(iv) Respiratory and circulatory systems are absent.
(v) Sexes are separate (unisexual) and females produce large number of eggs.
FAQs on Class 9 Science: Sample Question Paper- 7 (With Solutions)
| 1. What is the format of the Class 9 Science Sample Question Paper-7? |  |
| 2. How can I obtain the solutions for the Class 9 Science Sample Question Paper-7? |  |
| 3. What topics are covered in the Class 9 Science Sample Question Paper-7? |  |
| 4. Why is it important to practice sample question papers for Class 9 Science? |  |
| 5. Can the Class 9 Science Sample Question Paper-7 be used as a study resource for the final exam? |  |


















