Class 9 Science: Sample Question Paper Term I- 10 (With Solutions) PDF Download
Ques 1: In which physical state, water exists at?
(a) 100°C
(b) 0°C
Ans:
(a) Liquid and vapour.
(b) Solid and liquid.
Ques 2: What will happen to a plant cell if it is kept in a hypotonic solution?
Ans: It will swell due to entry of water by the process of osmosis.
Ques 3: A body is moving on a rough level road with a speed of 15 ms-1 along a given direction. Does any force is needed? Why?
Ans: Yes, a constant force is needed to maintain the motion. The force is needed to balance the force of friction acting due to the roughness of the road.
Ques: 4 Which chemical is mostly used to stain the animal cell in the laboratory?
Ans: Methylene blue
Ques: 5 State the function of chromosomes in a cell.
Ans: Chromosomes contain information for inheritance of features from parents to next generation in the form of DNA molecules.
Ques 6: A student burns a strip of magnesium metal in the flame. What will he observe?
Ans: The student will observe a white dazzling light.
Ques: 7 When sugar is dissolved in water, the resulting solution will appear as in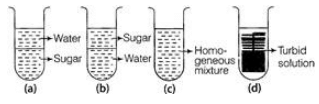
Ans: The resulting solution will appear as in C. It will appear as homogeneous.
Ques: 8 Reeta mixed starch with water, boiled the mixture well and stirred it. What did she observe?
Answer: Starch forms colloidal solution which is translucent.
Ques: 9 In an experiment to separate the compounds of a mixture of sand, common salt and ammonium chloride, which component will be removed by filtration?
Ans: Sand, as it is insoluble in water.
Ques: 10 What is the nature of motion in the following case?
Ans: It is non-uniform motion.
Ques: 11 Does the speedometer of a car measure its average speed?
Ans: No, the speedometer of a car does not measure its average speed. It measures only instantaneous speed.
Ques: 12 Rashi added 2 mL of barium chloride solution to 2 mL sodium sulphate solution in a test tube. What did she observe?
Ans: A white precipitate of BaSO4 settled down at the bottom.
Ques: 13 Take a test tube of good quality glass material and put a amount of water in it. Place a stop cork at the mouth of it. Now suspend the test tube horizontally by two strings or wires as shown in the figure.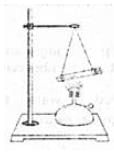
Heat the test tube with a burner until water vaporizes and the cork blows out. Write any two of your observations.
Ans: (i) Water converts into steam.
(ii) Steam exacts force on the cork and causes it to open.
Ques: 14 Identify the solute and solvent in : tincture of iodine.
Ans: Iodine is solute, alcohol is solvent.
Ques: 15 Draw diagram of stomata.
Ans: The labelled diagram of stomata are as shown below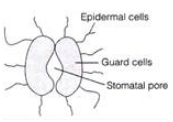
Ques: 16 How do you separate a mixture of sulphur, iron filings and salt? Give reason.
Ans: (i) Use Magnet it will remove iron filings.
(ii) Dissolve in Water Salt will be dissolved in water, sulphur will be residue.
(iii) Crystallization Salt will be obtained.
Ques: 17 Write difference between aerenchyma and chlorenchyma.
Ans: The difference between aerenchyma and chlorenchyma are
Aerenchyma: Parenchyma having large cavities to give buoyancy to aquatic plants to help them float.
Chlorenchyma: Chlorophyll containing parenchyma, which helps in photosynthesis is called chlorenchyma.
Ques: 18 (a) Students were instructed to add a few drops of iodine solution to each of the following samples.
The content turned blue-black in which sample?(b) Name the adulterant of arhar (tuvar) dal.
Ans: (a) In the samples of cooked rice and barley powder the content turned blue-black. (b) Metanil yellow.
Ques 19: To make a saturated solutions, 36g of sodium chloride is dissolved in 100g of water at 293 K. Find its concentration at this temperature.
Ans: 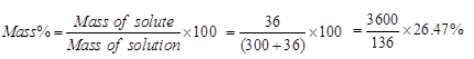
Ques 20: Name the most popular varieties of Aseel. What is the average weight of hen and cock of this variety?
Ans: Four varieties of Aseel breed of poultry are:
(i) Peela (golden red)
(ii) Yakub (black and red)
(iii) Nurie (white)
(iv) Kajal (black)
Average weight of hen of this variety is 3 to 4 kg and that of cock is 4 to 5 kg.
Ques 21: Define a solid. Give its main characteristics.
Answer: A solid may be defined as a state of matter which has a definite shape and fixed volume. Characteristic properties of solids
(i) Solids have fixed shape and fixed volume.
(ii) Solids cannot be compressed much.
(iii) Solids do not flow.
(iv) Solids have high densities. They are heavy.
Ques 22: What types of mixtures are separated by the technique of crystallization?
Ans: Crystallization method can be used to purify those mixtures which
(i) contain insoluble and/or soluble impurities.
(ii) are crystalline in nature.
(iii) either decompose or get charred (e.g., sugar) on heating to dryness, i.e., which cannot be separated by evaporation.
(iv) cannot be separated by filtration as some impurities are soluble.
Ques 23: Write the main function of each of the following cell components.
(a) Plasma membrane
(b) Mitochondria
(c) Chromosomes
(d) Ribosomes
(e) Lysosomes
(f) Golgi apparatus
Ans: (a) It regulates entry and exit of some materials in and out of the cell. It also prevents movements of some other materials.
(b) Also known as power house of the cell. Cellular respiration takes place in this organelle resulting in release of energy.
(c) Chromosomes contain genes. Genes are segments of DNA and are bearers of heredity characters. These are responsible for inheritance.
(d) Ribosomes are site of protein synthesis in a cell.
(e) These are also known as suicide bag of the cell. They contain digestive enzymes. They kill bacteria and remove worn out cell organelles.
(f) Golgi apparatus or Golgi body are the secretory organelle of the cell. They are also involved information of lysosomes.
Ques 24: What are the functions of parenchyma, collenchyma and sclerenchyma?
Ans: Parenchyma Main function is storage. Some parenchyma cells contain chlorophyll and help in photosynthesis. Collenchyma It is mechanical tissue, provides mechanical support and elasticity. When they contain chlorophyll they help in photosynthesis.
Sclerenchyma It is mechanical tissue, gives support to the plant body. Shell of nuts are hard due to presence of this tissue.
Ques 25: (a) What is cork? Mention its uses.
(b) How many types of elements are present in xylem?
Ans: (a) As root and stem grow older and increase in girth, peripheral tissue of root and stem become cork, cork cells are dead and do not have any intercellular spaces. Cork is protective in function. It is used for manufacture of sports goods, linoleum and for insulation.
(b) It consists of four types of elements:
(i) Tracheids
(ii) Vessels or Trachea
(iii) Xylem parenchyma
(iv) Xylem fibres
Ques 26: Satvik went on his bike from Delhi to Faridabad at 40 km/h and in the evening returned back at a speed of 60 km/h. What is his average speed for the entire journey? What is his average velocity?
Ans: The distance covered in forward journey from Delhi to Faridabad and back way journey from Faridabad to Delhi is exactly same i.e.,S1 = S2 = S (say) Again v1 = 40kmh-1 and v2 = 60kmh-1 Average speed 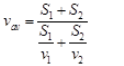
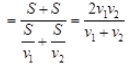
Ques 27: (a) Distinguish between G and g.
(b) What is the effect of shape of earth on value of g?
Ans: (a) The differences between gravitational constant(G) and acceleration due to gravity (g) are
| S. No. | Gravitational constant (G) | Acceleration due to gravity (g) |
| (i) | It is defined as the force of attraction between two objects of unit mass each separated by unit distance. | It is defined as the acceleration of an object freely falling under the action of force of gravity. |
| (ii) | It is an universal constant and its value is 6.673 x 10-11Nm2Kg-2 | It is a constant at a given place its value changes from place to place. Mean value of g on surface of earth is 9.8 ms-2 |
(b) The earth is not a perfect sphere. The radius of earth increases from poles to the equator. Hence, as per relation 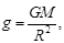 value of g i s greater at the poles and lesser at the equator.
value of g i s greater at the poles and lesser at the equator.
Ques 28: Two friends were playing 'catch the ball' in a park. 'A' was able to catch the ball comfortably without hurting himself, while 'B' was hurt every time he caught a ball. Finally B asked A how he could catch the ball with ease. A explained the phenomena to B. B thanked him.
(a) Why was A not hurt while catching the ball?
(b) Compare and contrast the values of A and B.
Ans: (a) A would lower his hands while catching the ball. This increased time of momentum change and ball exerted less force on the hands of A.
(b) B is inquisitive, accommodating and a keen observer. A is scientifically tempered, logical and has applicative mind.
Ques 29: (a) How are new varieties of poultry birds with desired traits produced?
(b) Mention any four desirable traits for which new varieties are produced.
(c) List the management practices that are common between dairy and poultry farming.
Or
(a) Write a short note on marine fisheries.
(b) Which factors should be taken into consideration for fish culture?
Ans: (a) By cross breeding between Indian (Indigenous) and foreign (exotic) poultry birds, desired traits are produced.
(b) Following are the four desirable traits for which new varieties are produced
(i) Number and quality of chicks.
(ii) Dwarf broiler parent for commercial chick production.
(iii) Summer adaptation capacity.
(iv) Tolerance to high temperature.
(c) The management practices that common between dairy and poultry farming are
(i) Proper arrangement for housing.
(ii) Proper arrangement for light.
(iii) Proper arrangement of nutrition.
Or
(a) India's marine fish area include 7500 km long coastline and deep sea beyond it. Marine fish are caught using many kinds of fishing nets from fishing boats. The yields are increased by locating large schools of fish, where large quantities of fishes can be found.
Popular marine fish varieties are pomphret, mackerel, tuna, sardines and Bombay duck.
Large schools are located by using satellites and echo sounders.
(b) The three important factors to be considered for fish culture are
(i) Topography, i.e., location of pond.
(ii) Water resources and their quality.
(iii) Soil quality.
Ques 30: Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
(c) Water at room temperature is a liquid.
(d) An iron almirah is a solid at room temperature.
(e) Ice at 273 K is more effective in cooling than water at the same temperature.
Or
(a) Dry ice is compressed under high pressure. What happens to it when pressure is released?
(b) Define (i) Melting point (ii) Fusion (c) Osmosis is a special kind of diffusion, Comment.
Ans: (a) Naphthalene undergoes sublimation easily i.e., the change of state of naphthalene from solid togas takes place easily. Thus, naphthalene balls disappear with time without leaving any solid.
(b) Gaseous particles possess high speed and large spaces between them. Particles of perfume diffuse into these gaseous particles at a very fast rate and reach our nostrils. This enables us to smell the perfume from a distance.
(c) At room temperature (25°C), water is a liquid because it has the following characteristic properties of liquid (i) At room temperature, water has no shape but has a fixed volume that is, it occupies the shape of the container in which it is kept. (ii) At room temperature, water flows.
(d) An iron almirah is a solid at room temperature (25°C) because (i) it has a definite shape and volume like a solid at room temperature. (ii) it is rigid as solid at room temperature.
(e) At 273 K, when ice melts, it absorbs the energy equal to the latent heat of fusion from the surroundings, therefore causes cooling effect.
Or
(a) On releasing the pressure, dry ice sublimes to vapour state without undergoing to liquid state.
(b) (i) Melting point The definite temperature at which a solid starts melting is called the melting point of that solid, e.g., melting point of ice is 0°C or 273.16 K.
(ii) Fusion The process of conversion of a solid into liquid state on heating is called fusion or melting.
(c) Diffusion is the process in which molecules of a substance move from the place of (their) higher concentration to the place of lower concentration. But during osmosis, the water (or solvent) molecules move from (their) higher concentration to the place of their lower concentration through a semipermeable membrane. Thus, osmosis is termed as a special kind of diffusion.
Ques 31: Name the tissue responsible for flexibility in plants. How would you differentiate it from other permanent tissues? Or Describe structure and function of different types of epithelial tissues.
Ans: The tissue responsible for flexibility in plants is collenchyma. The distinction of collenchyma from other permanent tissues is given below
| S. No. | Parenchyma | Collenchyma | Sclerenchyma |
| (i) | It consists of relatively unspecialized cells with thin walls. The cells of this tissue are living. | The cells of this tissue are living, elongated and irregularly thickened at the corners. | The cells of this tissue are dead. They are long and narrow as the walls are thickened due to lignin (a chemical substance which acts as cement and hardens them). |
| (ii) | The cells in this tissue contain large intercellular spaces. | There is very little intercellular space. | The walls of cells are so thick that there is no internal space inside the cell and between the cells. |
| (iii) | It provides support to plants and also stores food. | It allows easy bending in various parts of a plant (leaf, stem) without breaking. It also provides mechanical support to plants. | It provides strength to the plant parts. |
Or
The epithelial tissue are classified as follows
(i) Squamous epithelium: It consists of extremely thin and flat cells forming a delicate lining, e.g., the esophagus and lining of mouth. Skin epithelial cells arranged in many layers to prevent wear and tear, they are arranged in a pattern of layers, the epithelium called as stratified squamous epithelium.
(ii) Cuboidal epithelium: Cells are cube-like with rounded nuclei and form lining of kidney tubule and duct of salivary glands, where it provides mechanical support. It also helps in absorption, excretion and secretion.
(iii) Columnar epithelium: It consists of tall cells, which are pillar-like having elongated nuclei. It is found in inner lining of intestine and helps in absorption and secretion.
(iv) Ciliated columnar epithelium: The ciliated columnar epithelium tissue contains cilia in respiratory tract. The sec ilia move and their movement pushes the mucus forward to clear it. This type of epithelium is thus ciliated columnar epithelium.
(v) Glandular epithelium: Some-times epithelial cells acquire additional specializations as gland cells, which can secrete substances at epithelial surface. A portion of epithelial tissue folds in ward and multicellular gland is formed called glandular epithelium.
FAQs on Class 9 Science: Sample Question Paper Term I- 10 (With Solutions)
| 1. What is the importance of sample question papers for Class 9 Science Term I exam? |  |
| 2. How can solving sample question papers help in improving exam performance? |  |
| 3. Are the solutions provided for the sample question papers accurate? |  |
| 4. How can sample question papers help in understanding the weightage of different topics? |  |
| 5. Can solving sample question papers guarantee success in the Class 9 Science Term I exam? |  |


















