Coupling Constants - NMR Spectroscopy | Organic Chemistry PDF Download
Coupling Constant
When two protons couple to each other, they cause splitting of each other’s peaks. The spacing between the peaks is the same for both protons, and is referred to as the coupling constant or J constant.
- This number is always given in hertz (Hz), and is determined by the following formula:
J Hz = ∆ ppm x instrument frequency - ∆ ppm is the difference in ppm of two peaks for a given proton. The instrument frequency is determined by the strength of the magnet.
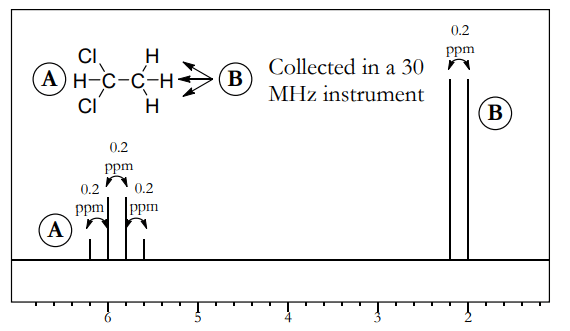
- Figure above shows NMR spectrum of 1,1-dichloroethane, collected in a 30 MHz instrument. This compound has coupling between A (the quartet at 6 ppm) and B (the doublet at 2 ppm).
- For both A and B, the distance between the peaks is equal. In this example, the spacing between the peaks is 0.2 ppm (for example, the peaks for A are at 6.2, 6.0, 5.8, and 5.6 ppm). This is equal to a J constant of (0.2 ppm • 30 MHz) = 6 Hz.
- Since the shifts are given in ppm or parts per million, you should divide by 106 . But since the frequency is in megahertz instead of hertz, you should multiply by 106 . These two factors cancel each other out, making calculations nice and simple.
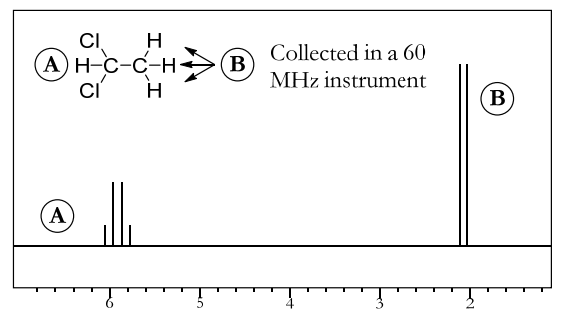
- Figure below shows the NMR spectrum of the same compound, but this time collected in a 60 MHz instrument.

- This time, the peak spacing is 0.1 ppm. This is equal to a J constant of (0.1 ppm • 60 MHz) = 6 Hz, the same as before. This shows that the J constant for any two particular protons will be the same value in hertz, no matter which instrument is used to measure it.
Important Points on Coupling Constant
- Coupling Constant is not dependent on strength of the external field.
- Multiplets with the same coupling constants may come from adjacent groups of protons that split each other.
- Coupling constant is a constant. It does not change in magnitude with change in instrument frequency.
- Increase in instrument frequency increases only the chemical shift of the nuclei, which increases the resolution of the spectrum.
- J can be either positive or negative, but only the absolute value is considered.

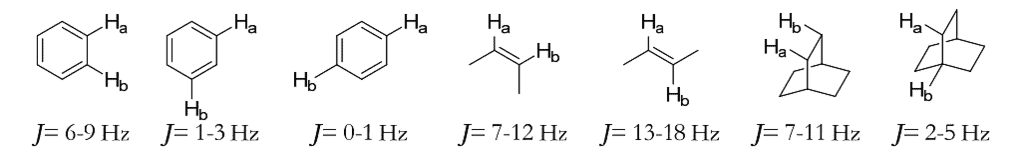
- The coupling constant provides valuable information about the structure of a compound. Some typical coupling constants are shown here.

Types of Coupling Constant
1. Based on the coupling partner:
- Homonuclear Coupling: Coupling between two nuclei of the same atom.
- Heteronuclear Coupling: Coupling between two nuclei of different atoms.
2. Based on the number of intervening bonds:
- One Bond Coupling (1J): Strong coupling, mainly heteronuclear.
- Two Bond Coupling (2J): Geminal coupling, high in magnitude.
- Three Bond Coupling (3J): Vicinal coupling, lower in magnitude, but highly significant for analysis.
3. Others: Specific couplings of higher order can be extremely useful
in NMR analysis.
- Ex: meta coupling, allylic coupling, W-coupling.
One Bond (1J) Coupling
Adjacent nuclei prefer to be in opposite spin states in the ground state. For a typical C—H bond, two peaks are observed in the 13C spectrum, due to the two shown transitions.
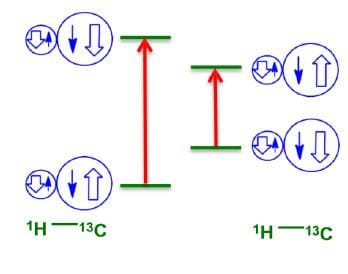
Some common one-bond coupling constants have been displayed below:
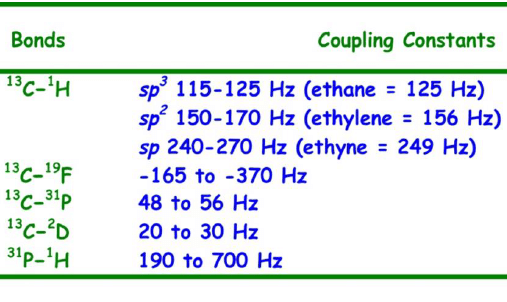
Two Bond (2J) Coupling
Commonly called as geminal coupling, these are usually smaller in magnitude than 1 bond coupling.

Some common 2 bond coupling constants:

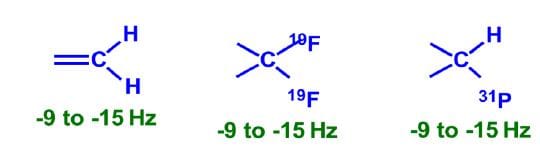
Variation in 2J Coupling
Geminal coupling increases in magnitude as the decreases, due to higher electron spin correlation.

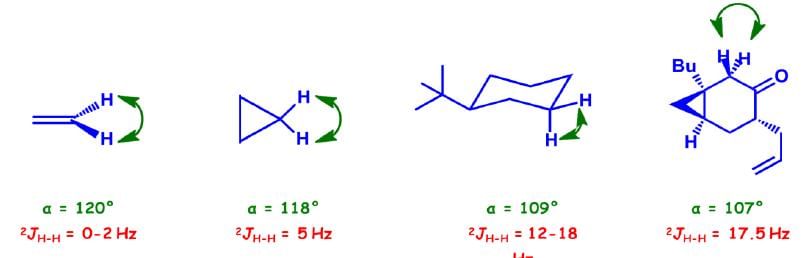
Three Bond (3J) Coupling
Commonly called vicinal coupling, these usually follow the (n+1) rule in simple aliphatic hydrocarbon chains. Nuclear and electronic spin interactions carry the spin information from one hydrogen to its neighbor.
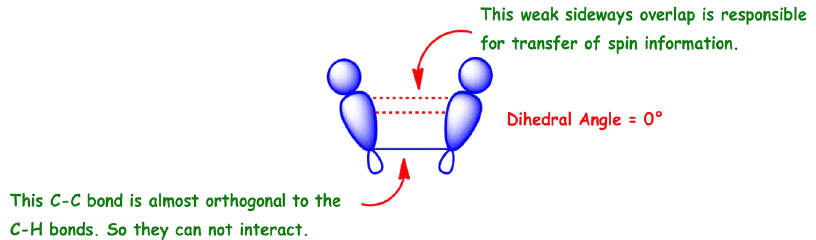
As a result, the best overlap occurs when the C—H bonds are at a dihedral angle of 0°.
Variation in 3J Coupling
The magnitude of vicinal coupling is directly related to the dihedral angle between the C—H bonds in question. The Karplus equation relates dihedral angle to the coupling constant between two vicinal hydrogen nuclei.


Coupling Constant of cis/trans alkenes:

Coupling Constant of
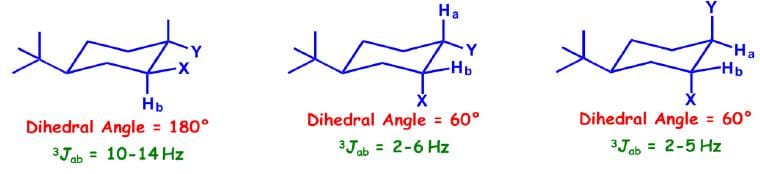
Cyclohexane systems:
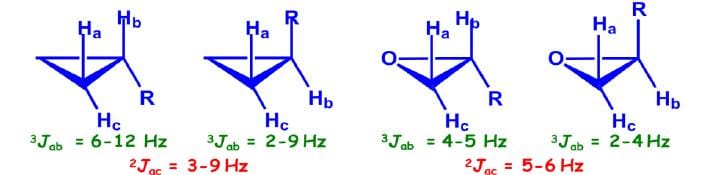
Coupling Constant of Cyclopropane/Epoxide Systems:
Jcis > Jtrans (unlike in alkenes)
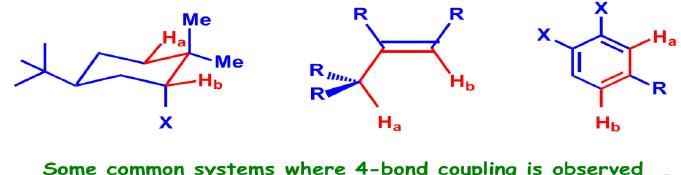
Long-Range Coupling
Very rarely, but often significantly, coupling takes place between two atoms separated by four bonds or more (nJ; n≥4). The common systems where this is exhibited are allylic systems, rigid bicyclic systems, and aromatic rings. Since these couplings are observed through a large number of bonds, a highly specific stereochemical arrangement is essential.
Stereochemical Nonequivalence:
- Usually, two protons on the same C are equivalent and do not split each other.
- If the replacement of each of the protons of a —CH2 group with an imaginary “Z” gives stereoisomers, then the protons are non-equivalent and will split each other.

|
35 videos|92 docs|46 tests
|
FAQs on Coupling Constants - NMR Spectroscopy - Organic Chemistry
| 1. What is a coupling constant in NMR spectroscopy? |  |
| 2. How is the coupling constant related to the number of bonds between coupled atoms? |  |
| 3. How does the coupling constant vary in 2J coupling? |  |
| 4. What is the coupling constant of cis/trans alkenes? |  |
| 5. What is the coupling constant of cyclohexane systems? |  |

|
Explore Courses for Chemistry exam
|

|