Cracking | Chemical Technology - Chemical Engineering PDF Download
6.1 Introduction
A critical observation of the overall refinery process block diagram indicates that the straight run gasoline (this is the gasoline obtained from the CDU) does not have good octane number (40 – 60) and needs to be upgraded to obtain the desired octane number (85 – 95).
Typically, cracking, reforming and isomerisation are regarded as the three most important processes that contribute towards upgradation of the octane number.
In this lecture, we present an overview of the cracking operation in the refinery.
Typically cracking involves the thermal or catalytic decomposition of petroleum fractions having huge quantities of higher molecular weight compounds. Since heat is required, typically cracking reactions are carried out in furnaces that are supplied with either fuel oil or fuel gas or natural gas or electricity as heat source. Cracking facilitates initiation, propagation and termination reactions amongst the hydrocarbon themselves. However, when steam cracking is carried out, in addition to the energy supplied by the direct contact of steam with the hydrocarbons, steam also takes part in the reaction to produce wider choices of hydrocarbon distribution along with the generation of H2and CO.
6.2 What is cracking?
- Cracking involves the decomposition of heavier hydrocarbon feedstocks to lighter hydrocarbon feed stocks.
- Cracking can be carried out to any hydrocarbon feedstock but it is usually applied for vacuum gas oil (VGO)
- Cracking can be with or without a catalyst.
- When cracking is carried out without a catalyst higher operating temperatures and pressures are required. This is called as thermal cracking. This was the principle of the old generation refineries.
- Now a days, cracking is usually carried out using a catalyst. The catalyst enabled the reduction in operating pressure and temperature drastically.
6.3 Cracking chemistry
- Long chain paraffins converted to olefins and olefins
- Straight chain paraffins converted to branched paraffins
- Alkylated aromatics converted to aromatics and paraffins
- Ring compounds converted to alkylated aromatics
- Dehydrogenation of naphthenes to aromatics and hydrogen
- Undesired reaction: Coke formation due to excess cracking
- Cracking is an endothermic reaction
Therefore, in principle cracking generates lighter hydrocarbons constituting paraffins, olefins and aromatics. In other words, high boiling low octane number feed stocks are converted to low boiling high octane number products.
6.4 Operating conditions
- These very much depend upon the feed stock and type of cracking (thermal /catalytic ) used.
- Cracking is a gas phase reaction. Therefore, entire feedstock needs to be vaporized.
- It was observed that short reaction times (to the order of 1 – 3 seconds only) provide good quality product and less coke formation.
- For vacuum gas oil, thermal cracking requires operationg at 600°C and 20 atms gauge pressure.
- For vacuum gas oil, catalytic cracking is usually carried out at 480°C and 0.7 – 1 atms gauge pressure.
6.5 Catalyst
- Acid treated silica-alumina was used as catalyst.
- 20 – 80 mesh size catalysts used for FCCR and 3 – 4 mm pellets used for MBRs
- During operation, poisoning occurs with Fe, Ni, Vd and Cu
6.6 Process technology
The process technology consists of two flowsheets namely the cracking coupled with main distillation column and stabilization of naphtha
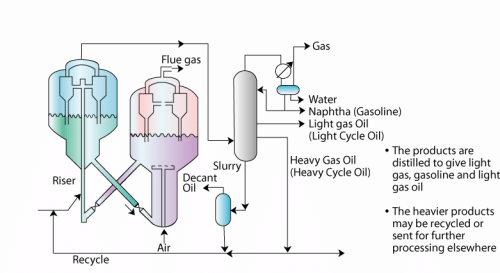
- Feed enters the cracking reactor.
- Now a days, fluidized catalytic cracking (FCC) reactors are used.
- The cracked product from the reactor enters a main distillation column that produces unstabilized naphtha, light gas oil, heavy gas oil, slurry and gas.
- Old generation refineries used moving bed reactors
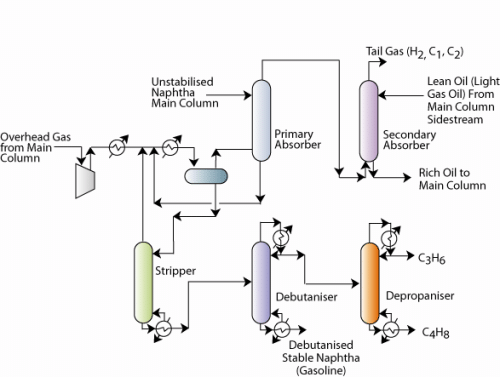
Figure6.2 Naphtha stabilization flow sheet
- The slurry enters a phase separation unit which separates decant oil and a heavier product. The heavier product is recycled back to the cracking reactor.
- The unstabilized naphtha subsequently enters a unsaturates gas plant
- In the unsaturates gas plant, the gas obtained from the main distillation column is sent to a phase separator. The phase separator separates lighter hydrocarbons from heavier hydrocarbons.
- The naphtha obtained is unstabilized, as it consists of various hydrocarbons. It is therefore subjected to stabilized by continued processing.
- The gas leaving the primary absorber is sent to a secondary absorber where light gas oil from main distillation column is used as a absorbent to further extract any absorbable hydrocarbons into the light gas oil. Eventually, the rich light gas oil enters the main distillation column (not shown in the figure a).
- The naphtha generated from the phase separator is sent to stripping to further consolidate and stabilize naphtha.
- The stabilized naphtha is further subjected to distillation in debutanizer and depropanizer units.
- The debutanizer unit removes butanes and lower hydrocarbons from the naphtha. The naphtha obtained as bottom product in the debutanizer is termed as debutanized stable naphtha or gasoline.
- The butanes and other hydrocarbons are sent to a depropanizer unit where butanes are separated from propanes and other lighter hydrocarbons. Thus, butanes are obtained as lower product and propanes along with other lighter hydrocarbons are obtained as the top product in the depropanizer unit.
- The phase separator is also fed with the unstabilized naphtha. The unstabilized naphtha from the main column is first fed to a primary absorber to absorb heavier hydrocarbons in the gas stream emanating from the phase separator.
Fluidized catalytic cracking reactor (FCCR) : (Figure 6.3)
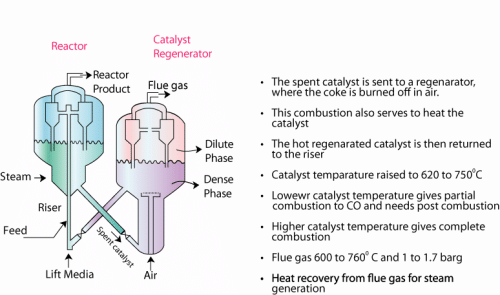
Figure6.3 Fluidized Catalytic Cracking Reactor ..........................
- Another issue for the FCCR is also to regenerate the catalyst by burning off the coke in air.
- Therefore, the reactor unit should have basically two units namely a reactor (FCCR) and a catalyst regenerator (CR).
- The FCCR consists essentially of two important components in a sophisticated arrangement. These are the riser and the cyclone unit assembled in a reactor vessel.
- Riser: In the riser (a long tube), the feed is allowed to get in contact with the hot catalyst. The hot catalyst is enabled to rise through lift media in the riser. The lift media is usually steam or light hydrocarbon gas.
- The riser contact time is about 250 milliseconds.
- The riser is eventually connected to cyclone units.
- The cyclone units receive the catalyst and finished product. The catalyst that enters the cyclone unit is fully coked and needs to be sent to a regenerator to regain its lost activity.
- After cyclone operation (which separates the hydrocarbon vapors and catalyst as a solid fluid operation), the catalyst falls down to the vessel that houses the riser and cyclone units.
- The catalyst in the vessel is subjected to stream stripping in which direct contact with steam is allowed to remove hydrocarbons from the catalyst surface.
- The basic principle of the FCCR is to enable the fluidization of catalyst particles in the feed stream at desired pressure and temperature.
Catalyst regenerator (CR)
- The spent catalyst which is relatively cold enters the regenerator unit.
- Here air enters the vessel through a sparger set up.
- The catalyst is subsequently burnt in the air. This enables both heating the catalyst (which is required to carry out the endothermic reaction) and removing the coke so as to regain the activity of the coke.
- The catalyst + air after this operation will enter the cyclone separator unit. Unlike the FCCR, the CR does not have a riser. Therefore, air enters a dense phase of catalyst and also enables the movement of the catalyst to a dilute phase of catalyst + air
- The cyclone separators separate the flue gas and catalyst as a solid fluid operation.
- The activity regained catalyst is sent to the riser through a pipe.
- During this entire operation, the catalyst temperature is increased to 620 – 750°C
- The flue gas is obtained at 600 - 760°C and is sent for heat recovery unit to generate steam.
|
69 videos|121 docs
|
FAQs on Cracking - Chemical Technology - Chemical Engineering
| 1. What is chemical engineering and why is it important? |  |
| 2. What are the typical job roles for chemical engineers? |  |
| 3. How does chemical engineering contribute to sustainability? |  |
| 4. What are the challenges faced by chemical engineers in their profession? |  |
| 5. What are the future prospects for chemical engineers? |  |

|
Explore Courses for Chemical Engineering exam
|

|

















