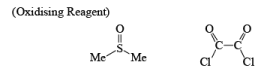
DMSO Based Oxidizing Reagent & Introduction to Reducing Reagents | Organic Chemistry PDF Download
DMSO Based oxidizing reagents
Swern Oxidation:
The mixture of oxallyl chloride and DMSO treated at low temperature with alcohol then TEA oxidation of alcohol takes place.
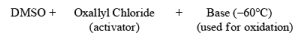
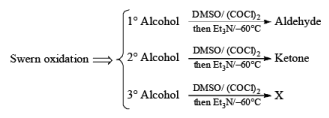
Mechanism of Swern Oxidation:
Step 1: Active form of DMSO in Swern Oxidation or intermediate of Swern Oxidation Chloro sulphonium ion.
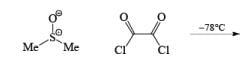
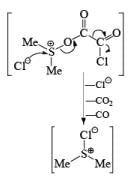
Step 2.
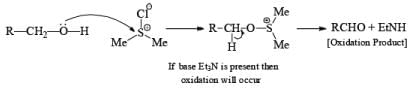
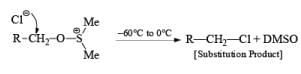
If base is not present then subst itution will occ ur
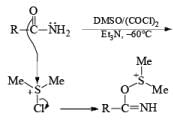
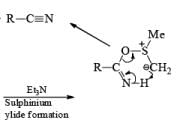
Note: Swern oxidation can also be used for the Amide oxidation. It will oxidize the amide into cyanide.
REDUCING REAGENTS

1. Heterogeneous Catalytic Hydrogenation (a) Heterogeneous (b) Homogeneous
2. Nucleophilic & Electrophilic Reduction
3. Dissolving Metal Based (Birch Reduction)
4. Tin hydrides (TBTH)
5. Diimide Reagents
1(a): Heterogeneous Catalytic Hydrogenation
Metal : {Pd, Pt, Rh, Ni}
Support for metal: Charcoal, Alumina, Silica Solvent: MeOH, EtOH, EtOAc, AcOH, Et2O, C6H14.
Reactivity Order of catalyst: H2-RaNi > H2PdC > H2- Pt-C > H2-Rh/Al2O3
Reactivity Order of function group: RNO2 > RCOCl > RC º R > RCH = CH2 > RCOR > RCN
Hydrogenation of carbon-carbon double bonds is frequently carried out in the presence of a heterogeneous metal catalyst generally proceeds under mild conditions. Selective reduction of a double bond in the presence of other unsaturated groups is usually possible, except when the compound contains triple bonds, nitro groups, or an acyl halide. The mechanism of heterogeneous hydrogenation of carbon-carbon double involves:
1. Dissociative chemisorption of H2 on the catalyst
2. Coordination of the alkene to the surface of the catalyst
3. Addition of the two hydrogen atoms to the activated R-bond in a syn-manner.
Catalyst Selection: For low-pressure hydrogenations (1-30 atm), Pt, Pd, Rh, and Ru are used. The reactivity of a given catalyst decreases in the following order: Pt > Pd > Rh – Ru > Ni. For high-pressure hydrogenations (100-300 atm), Ni is usually the metal of choice.
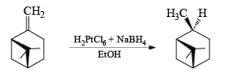
Platinum, prepared by reduction of PtO, (Adams catalyst) with H2, is pyrophoric. Usually 0.1-1% of the catalyst is employed. A more convenient procedure for the preparation of Pt is by reduction of chloroplatinic acid with NaBH4 in ethanol.
H2 PtCl6 . 6H 2O + EtOH + NaBH4 —→ Pt - catalyst
Palladium is available as a metal deposit on the surface of an inert support such as carbon.
Nickel is used for high-pressure hydrogenations. Supported-Ni catalysts such as Raney-Ni and Ni-Boride are employed for hydrogenolysis of the G-S bonds in thioacetal. Raney-Ni is prepared by treating nickel-aluminum alloy with NaOH. Freshly prepared Raney-Ni absorbs much of the H, produced in the reaction.
NiAl2 + 6NaOH + H2O —→ Ni(ppt) + 2Na3AlO3 + 3H2
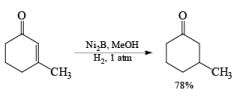
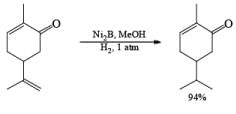
Ni-Boride, readily prepared by reduction of Ni(OAc)2 with NaBH4, is more reactive than Raney-Ni., treatment of NiCl2 with NaBH4 in methanol followed by heating yields a stable suspension of Ni2B, which catalyzes the regioselective 1,4-reduction of a, b-unsaturated aldehydes and ketones.
NiCl2 6H2O a H4 in MeOH —→ NiB suspension (store up to six months at 25 C)
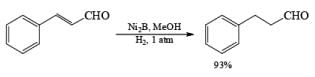
Solvent: The activity of a given catalyst generally is increased by changing from a neutral to a polar to an acid solvent. EtOAc, EtOH, and HOAc are the most frequently used solvents for low-pressure hydrogenations.
1(b). Homogeneous Catalytic Hydrogenation (Wilkinson’s catalyst)
Chlorotris (triphenylphosphine)rhodium (Wilhnson’s catalyst) is among the most efficient catalysts and permits hydrogenation in homogeneous solution. The Rh complex is readily prepared by heating rhodium chloride with excess triphenylphosphine in ethanol.
RhCl 3 · H2O + Ph3P (excess) ® (Ph3P)3RhCl [ Wilkinson's catalyst]
Characteristic features of this Rh-catalyst include:
I. Hydrogenation of double bonds via syn-addition of H2
II. Cis-double bonds are hydrogenated faster than trans-double bonds,
III. Terminal double bonds are hydrogenated more rapidly than more substituted double bonds
IV. Less isomerization of double bonds,
V.Little hydrogenolysis of allylic or benzylic ethers and amines, and
VI. R – C º N, R - NO2, R-Cl, RCOOH, RCOOR', and R2C=O are not reduced.
VII. Relative reactivity of alkynes & alkenes for hydrogenation using Wilkinson’s catalyst:
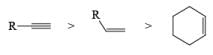
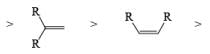
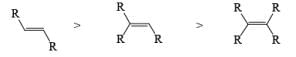
2. Nucleophilic & Electrophilic Reduction
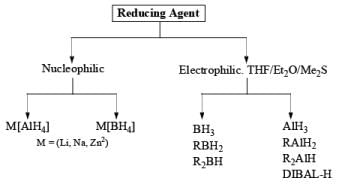
Nucleophilic Reducing Agents: The reduction takes place through hydride transfer. The reaction medium (Et2O, THF, ROH, H2O) The reactivity order of substrates is: RCHO > R2CO > RCO2R’ > RCONR2 > RCO2H.
ALUMINIUM HYDRIDES
Lithium Aluminum Hydride-LiAlH4
Lithium aluminum hydride (LAH) is a powerful reducing agent but is not very chemo-selective. It must be used in nonprotic solvents such as Et2O or THF. The reagent is usually used in excess, with only three hydrides of LiAlH4 being utilized.
Stoichiometry of Hydrides in LiAlH4 Reductions
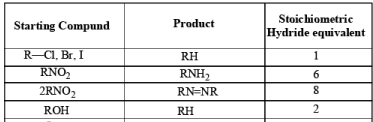
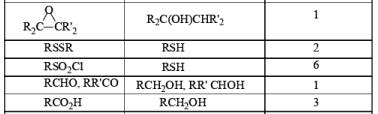
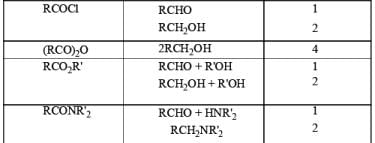

The reactivity of LAH may be tempered by the addition of dialkyl amines. For example, both aliphatic and aromatic carboxylic esters are reduced to the corresponding aldehydes by LAH in the presence of diethylamine at room temperature.
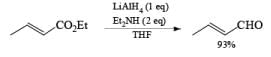
Lithium Trialkoxyaluminum Hydride-Li[AiH(OR)3]
The reactivity and selectivity of LAH can be modified by replacing three of its hydrides with t-butoxy or ethoxy group. The resulting reagents are less reactive but more selective than LAH and are best prepared just prior to use in situ.
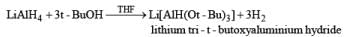
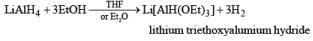
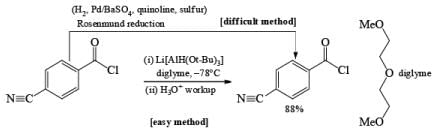
Lithium tri-t-butoxyaluminunz hydride readily reduces aldehydes and ketones to the corresponding alcohols and reduces acid chlorides to aldehydes. Epoxides, esters, carboxylic acids, tert-amides, and nitriles are not, or only slowly, reduced. Thus, the reagent may be used for chemoselective reduction.
Note that before lithium trialkoxyaluminum hydrides became available, acid chlorides were converted to aldehydes using the more tedious
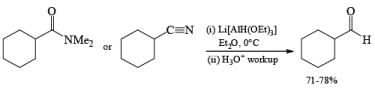
Lithium triethoxyaluminum hydride is a more powerful reagent which reduces tert-amides and nitriles to the corresponding aldehydes.
Sodium Bis(2-methoxyethoxy)aluminum Hydride-Na[AlH2(OCH2CH2OCH3)2]
Sodium bis(2-methoxyethoxy)aluminum hydride, or Red-Al, is a versatile, commercially available reducing agent. It is thermally more stable than LAH and may be used in aromatic hydrocarbon as well as in ether solvents. Overall, the reducing properties of Red-A1 are similar to those of LAH (reductions of aldehydes, ketones, esters, etc.).
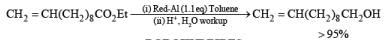
BOROHYDRIDES
The nucleophilic borohydrides-sodium borohydride [NaBH4], lithium borohydride [LiBH4], zinc borohydride [Zn(BH4)2], lithium- and potassium trialkylborohydrides [Li-, K-R3BH], and sodium cyanoborohydride [NaBH3CN]—exhibit, depending on the metal cation and the ligands, characteristic reducing properties toward functional groups. All borohydrides reduce aldehydes and ketones; reduction of esters and carboxylic acids to primary alcohols requires specific reagents.
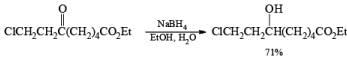
Lithium Borohydride
Lithium borohydride is a commercially available, solid reagent that rapidly decomposes when exposed to moist air. It is conveniently prepared from NaBH4 and LiBr in Et2O or in THF. The NaBr precipitates as it is formed, and the clear supernatant solution is used as such for reductions after determination of its hydride concentration
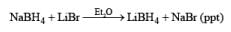
The Li’ cation is a stronger Lewis acid than the Na+ cation. Li+ coordination with the carbonyl group enhances the electrophilicity of the carbonyl carbon, thereby facilitating hydride transfer. Lithium borohydride is a more powerful reducing agent than sodium borohydride: it reduces esters to primary alcohols but is unreactive towards amid

Zinc Borohydride
Zinc borohydride is not commercially available; it is prepared from anhydrous ZnC12 and NaBH4 solutions in ether solvents. The chloride-fice supernatant solutions then are used as required.
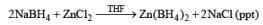
Zinc borohydride has some interesting properties: it is less basic than NaBH4 and thus it is especially suitable for the reduction of base-sensitive compounds. Also, the zinc cation has a better coordinating ability than either Na+ or Li+, making Zn(BH4)2 often the reagent of choice for chelation-controlled, stereoselective reductions of acyclic ketones.
Besides aldehydes and ketones, Zn[BH4]2 reduces aliphatic esters and carboxylic acids. Moreover, it is an effective hydroborating agent for alkenes, dienes, and alkyn.
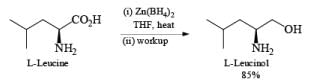
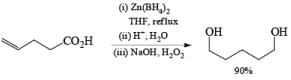
Lithium or Potassium Trialkylborohydride
The presence of three alkyl groups in lithium trialkylborohydrides imparts increasing nucleophilicity to the hydride, making them more powerful reducing agents than lithium borohydride itself. Aldehydes, ketones, and esters are rapidly and quantitatively reduced to the corresponding alcohols even at –78°C. The most frequently used trialkylborohydrides are lithium triethylborohydride (Superhydride) and lithium and potassium tri-seebutylborohydride (L- and K-Selectride). Lithium triethylborohydride (LiEt3BH) is a super-nucleophile that reduces primary alkyl bromides, and tosylates more effectively to the corresponding hydrocarbons than does LiAlH4. Epoxides are readily cleaved to give alcohols by attack of the hydride at the less substituted carbon.
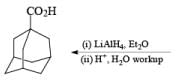
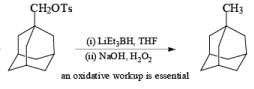
Sodium Cyanoborohydride-NaBH3CN
Because of the presence of the electron withdrawing cyano group, NaBH3CN is less nucleophilic and hence is more selective than NaBH4.
The utility of NaBH3CN as a reducing agent is greatly enhanced by its stability toward low pH (stable to pH 3).
Thus, the reagent permits reductions under conditions that would rapidly hydrolyze NaBH4. NaBH3CN is soluble in a variety of solvents: H2O, ROH, THF, but it is insoluble in Et2O and hydrocarbon solvents. Under neutral conditions in H2O or MeOH, reduction of RCHO and R2CO is negligible. However, in acidic solution, carbonyl reduction does occur (protonated carbony1 group)
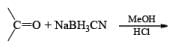
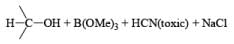
NaBH3CN is a chernoselective reducing agent. For example, it is possible to selectively reduce an aldehyde group in the presence of a keto group or a keto group in the presence of an ester group using NaBH3CN. Even under diverse reaction conditions, functional groups such as RCONH2, –C º N, and -NO2 are inert toward the reagent
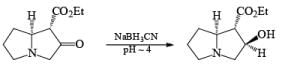
Reductive Amination with NaBH 3C ≡ N
Since the reduction of an iminium salt by NaBH3CN occurs more readily than the reduction of a carbonyl group, NaBH3CN is the reagent of choice for the reductive amination of aldehydes and ketones. The reaction entails condensation of the carbony1 compound with NH3, RNH2, or R2NH at pH 5 to 8 to give the corresponding iminium salts. These are reduced in situ with NaBH3CN to furnish primary, secondary, or tertiary amines, respectively
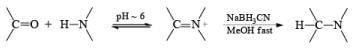
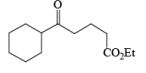
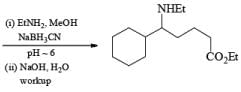
Sodium triacetoxyborohydride Na[BH(OAC)3] and hydrogenation (H2, Pd/C) are used as alternatives to sodium cyanoborohydride for the reductive amination of carbonyl compounds. Also, Zn [BH4]2 is a particularly effective agent for the reductive amination of α, β-unsaturated aldehydes and ketones.
|
35 videos|92 docs|46 tests
|
FAQs on DMSO Based Oxidizing Reagent & Introduction to Reducing Reagents - Organic Chemistry
| 1. What is the role of DMSO in an oxidizing reagent? |  |
| 2. What are some examples of DMSO-based oxidizing reagents? |  |
| 3. How does DMSO-based oxidation differ from other oxidation methods? |  |
| 4. What are the benefits of using reducing reagents in organic chemistry? |  |
| 5. Can you provide some examples of commonly used reducing reagents? |  |

|
Explore Courses for Chemistry exam
|

|


















