Diagram Label Completion: Practice Test - 5 | Reading for Academic IELTS PDF Download
Directions: Answer the Diagram label completion questions from the passage as follows:
It is essential when conducting this experiment to wear safety goggles. This experiment is divided into four distinct sections. The first, the reaction stage, is when a glass beaker is placed on top of a tripod, and 20cm of dilute sulphuric acid poured into it. The acid is then heated. When it is almost boiling, a small quantity of copper oxide powder is added to the beaker. The mixture is then stirred with a glass spatula until the copper oxide has dissolved. This process is then repeated until 1g of powder has been added to the sulphuric acid. The heat is then removed from the beaker and the solution allowed to cool. The second stage is the filtration stage and, as the name suggests, is where a filter and conical flask are used to remove any copper oxide that has not reacted. A clear copper sulphate solution will be left in the glass dish. The third stage is where heat is applied to the copper sulphate solution in order to concentrate the solution: the concentration stage. The final crystallization stage happens when the solution begins to cool, and pure copper sulphate crystals start to form.
Q. The diagram below shows how copper sulphate can be made using simple laboratory equipment. Choose NO MORE THAN THREE WORDS AND/OR A NUMBER from the passage for each answer. Label the diagram.
Write your answers for 1–6 on your answer sheet.
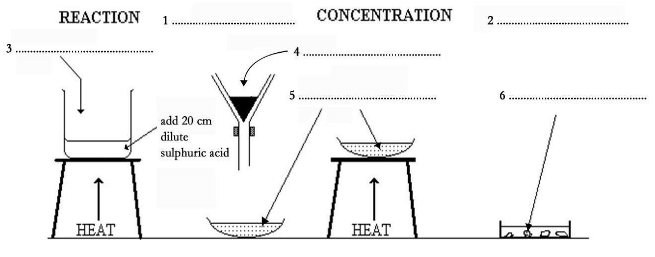
Solution of 1:
"Filtration"
The second stage is the filtration stage and, …..
Solution of 2:
"Crystallization"
The final crystallization stage happens when the solution begins to cool, and pure copper sulphate crystals start to form.Answers 1 and 2 can be understood from the mentioned information that defines stages. The two stages( out of four) are mentioned in the diagram. As it is a list, similar information is required. The guiding words for you are: first, second, third and final.
Solution of 3:
"Copper oxide powder"
When it is almost boiling, a small quantity of copper oxide powder is added to the beaker.
The process of heating is mentioned in the diagram, and the arrow at Question 3 indicates the addition of an element.
Solution of 4:
"Remove copper oxide / filter copper oxide"
The second stage is the filtration stage and, as the name suggests, is where a filter and conical flask are used to remove any copper oxide that has not reacted.
The dimensions of the flask and the shaded portion in the flask indicate the residue in the filter.
Solution of 5:
"Copper sulphate solution"
A clear copper sulphate solution will be left in the glass dish. The third stage is where heat is applied to the copper sulphate solution in order to concentrate the solution; the concentration stage.
The shape of the dish, the heat are indicators of what is obtained next.
Solution of 6:
"Copper sulphate crystals"
The final crystallization stage happens when the solution begins to cool, and pure copper sulphate crystals start to form.
The keyword ‘final’ guides you to the answer and the shape of the contents also lead you to the word ‘crystals’.
|
27 videos|111 docs
|
FAQs on Diagram Label Completion: Practice Test - 5 - Reading for Academic IELTS
| 1. What is the IELTS exam? |  |
| 2. How can I prepare for the IELTS exam? |  |
| 3. What is the difference between the IELTS Academic and IELTS General Training modules? |  |
| 4. How is the IELTS exam scored? |  |
| 5. What is the validity period of the IELTS exam results? |  |

|
Explore Courses for IELTS exam
|

|

















