Different Atomic Models | Physics for Grade 10 PDF Download
Introduction
- The understanding of the structure of an atom has changed over time
- The best model of an atom is the one that can explain the evidence of experiments best
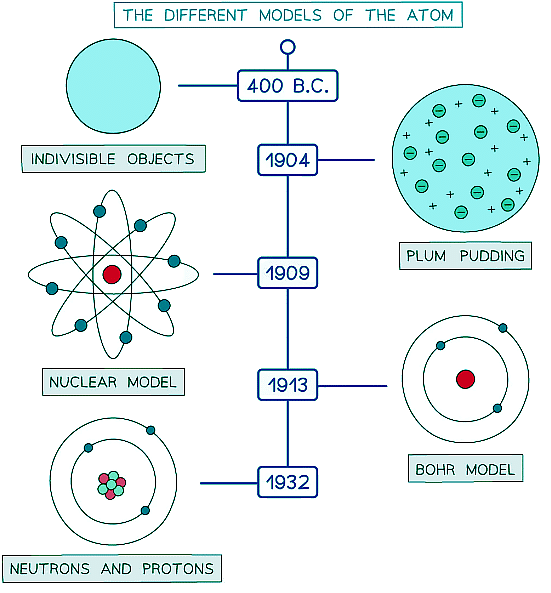
- The image below shows a timeline of the different models of the atom
 Scientific models are used to explain observations. Models of the atom have changed and improved throughout history
Scientific models are used to explain observations. Models of the atom have changed and improved throughout history
Comparing Atomic Models
- Rutherford’s alpha scattering experiment led to a change in the atomic model
- The Plum Pudding model was replaced by the nuclear model
- The nuclear model could explain why the alpha particles bounced back from the gold foil
Differences between the Plum Pudding and Nuclear model Table

Solved Example
An experiment, designed to investigate the ‘plum pudding’ model, involved firing alpha particles at a thin gold foil. If the ‘plum pudding’ model was correct, then most of the alpha particles would go straight through the gold foil. A few would be deflected but by very small angles.
The results of the experiment were unexpected. Although most of the alpha particles did go straight through the gold foil, about 1 in every 8 000 was deflected by very large angles. Using the nuclear model, the scientist Ernest Rutherford devised an equation to predict the proportion of alpha particles that would be deflected through various angles.
The results of the experiment were the same as the predictions made by Rutherford.
Explain:
(a) Why this experiment led to a new model of the atom, called the nuclear model, which replaced the ‘plum pudding’ model.
(b) Why it is important that the experimental results and the predictions are the same.
(a) The experimental results of the gold foil experiment could not be explained using the plum pudding model
Therefore, the plum pudding model was disapproved and a new model, the nuclear model, was devised to match the results
(b) If the predictions are correct, then this proves that the nuclear model is correct
|
124 videos|149 docs|37 tests
|

|
Explore Courses for Grade 10 exam
|

|