Dobereiner's Triads, Octaves & Mendeleev's Periodic Table | Physical Science for High School - Grade 9 PDF Download
| Table of contents |

|
| Introduction |

|
| Dobereiner Triads Rule |

|
| Newlands Law of Octave |

|
| Mendeleev's Periodic table |

|
Introduction
- Before the beginning of the eighteenth century, when there were only 30 elements known, it was easier to study and remember their properties.
- In later years, when the number of elements discovered increased, it became difficult to study them.
- So, scientist fell the need for a simple method to facilitate the study of the properties of various elements and their compounds.
- After numerous attempts, they got success & elements were arranged so that similar elements were grouped together, and different elements were separated.
- This arrangement of elements is known as the classification of elements which led to the periodic table formation.
Note: Periodic table may be defined as the arrangement of all the known elements according to their properties in such way that the elements of similar properties are grouped together in a tabular form.
Earlier attempts of classification of elements (development of periodic table):
Earlier attempts to classify the elements resulted in the grouping as metals and non-metals. Later on, they were classified on the basis of their atomic masses.
Dobereiner Triads Rule
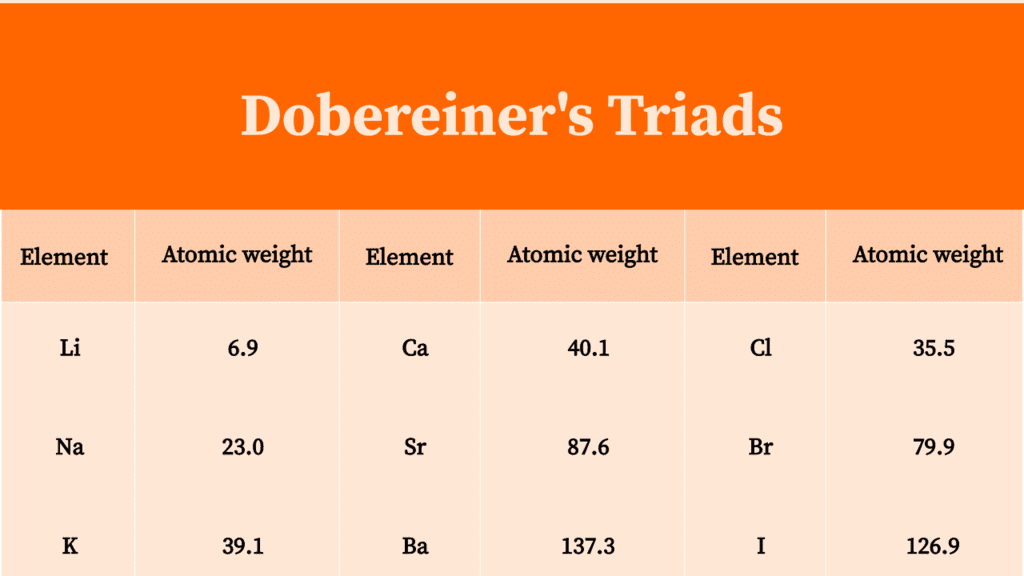
- In 1817, Johann Wolfgang Dobereiner, a German chemist, arranged the elements in the group of three elements and in a manner that the atomic mass of middle element was roughly the average of the atomic masses of the other two elements of the triad.
Example: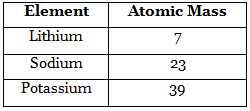
- Average of the atomic masses of Lithium and Potassium is
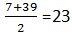 . Only three triads could be arranged in this manner at that time.
. Only three triads could be arranged in this manner at that time.
They were: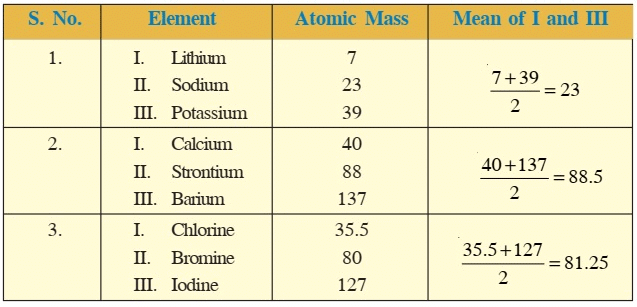
Short Comings of Dobereiner Triad's Rule
- This classification was not found satisfactory as it could be applied to the limited number of elements.
Nowadays some more triads have been made, they are: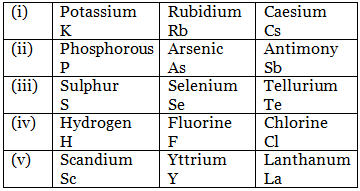
- For a Dobereiner's triad, all the three elements should belong to the same group, and the difference in atomic number should be 8 or 18.
Newlands Law of Octave
- In 1866, J.A.R. Newlands correlated the chemical properties of the elements with the increasing order of atomic masses. i.e. to place the element having the lowest atomic mass (H) at the first position and end with the element having the highest atomic mass. (Thorium which was 56th known element at that time).
Definition
- When the elements are arranged in order of their increasing atomic masses, every eighth element has properties similar to those of the first elements like the eighth note of an octave in music.
- Thus according to this law, the physical & chemical properties are repeated after an interval of eight elements.
This is similar to eight notes of an octave on a musical scale shown below: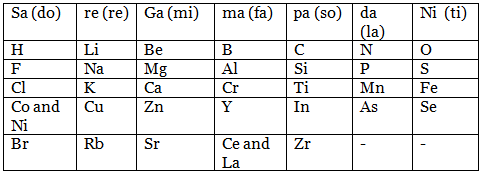
- The properties of Lithium are similar to that of 8th element, i.e. Na, Be is similar to Mg and so on.
Limitations
- Law of octaves was applicable only up to calcium. It worked well with lighter elements only.
- At that time only 56 elements existed in nature, but later several elements were discovered which cannot be kept in the periodic table as per this law. Their properties were not in accordance with the law of octaves.
Law of Octaves
- In order to fit element into his table, Newlands adjusted two elements in the same column. For example, cobalt and nickel were placed in the same position and in the same column as fluorine, chlorine and bromine.
- Iron which resembles cobalt and nickel in properties were placed far away from these elements.
Note: After the discovery of inert gases, included in the periodic table it becomes the eighth element from alkali so this law has to be dropped out.
Mendeleev's Periodic table
- In the year 1861, D Mitri Ivanovich Mendeleev arranged all the known elements (63 elements) in the form of a table in which elements were arranged in the increasing order of their atomic mass and also on the similarities of chemical properties.
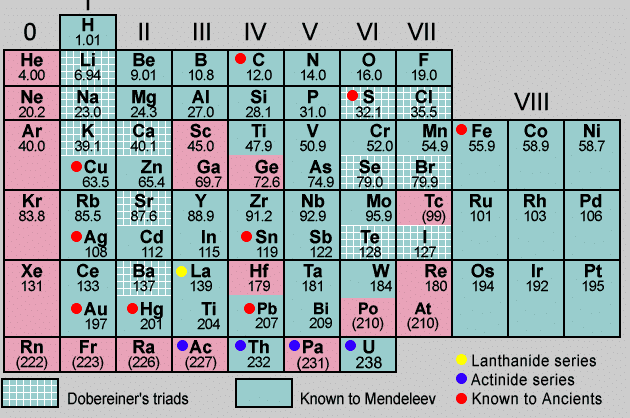 Mendeleev's Periodic Table
Mendeleev's Periodic Table
- The arrangement of elements was based on their physical and chemical properties and also on the formulae of the compounds they formed with oxygen and hydrogen.
- He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements.
- The table which classifies the elements in such a way that elements having similar properties are placed in the same vertical column or group is known as the periodic table.
- Mendeleev's periodic table had six periods and eight groups, as shown in the table, he arranged all the elements horizontally in the order of their increasing atomic masses and vertically according to their similarities in properties.
- Each group was further subdivided into two subgroups A & B.
Achievements of the Mendeleev's Periodic Table
1. Systematic Study of the Elements
- All the elements, in general, were arranged systematically in increasing order of their atomic masses.
- This arrangement helped to study the properties of various elements.
- If the nature of the element present in a group is known, it becomes easier to predict or guess the expected properties of other elements.
2. Prediction of New Elements
- Mendeleev predicted the properties of some unknown elements and left gaps for these elements to be filled as and when discovered.
Example: Scandium, Gallium and Germanium were not known at that time, but Mendeleev already named these elements as eka-boron, eka-aluminium and eka-silicon.
Note: When these elements were later on discovered, they were found to have more or less similar properties as predicted by Mendeleev.
3. Position of Noble Gases
- When noble gases were discovered, they were placed in a new group without disturbing the existing order.
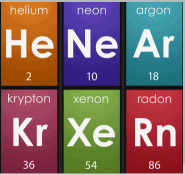 Position of Noble Gases in Periodic table
Position of Noble Gases in Periodic table4. Correction of Atomic Masses
- Atomic masses of several elements were corrected on the basis of the periodic table.
Example: Atomic mass of Beryllium was corrected from 135 to 9. - Mendeleev predicted that the atomic mass of gold is incorrect. Later on, it was found to be 197. Similarly, atomic masses of Indium, Uranium and Platinum were also corrected.
Drawbacks of Mendeleev's Periodic Table
(i) Position of Hydrogen is uncertain because it resembles with I A group alkali metals elements and VII A (halogens) group elements.
(ii) Isotopes
- Isotopes of an element have similar chemical properties but different atomic masses.
(iii) Position of Isotopes
- Since the basis of the periodic table was increasing atomic mass. So isotopes should be placed separately, but no separate place was given to isotopes.
(iv) Anamolus Pairs of Certain Elements
- Certain elements were not arranged according to their increasing atomic mass.
Example:
(a) Argon (Atomic mass 39.9) was placed before potassium (atomic mass 39.0)
(b) Cobalt (58.95) before Nickel (58.70)
(c) Tellurium (127.6) before Nickel (126.9)
(d) Thorium (232) before Protactimum (231)
(v) Similar Elements were Placed in Different Groups
- Example:
(a) Silver and thallium
(b) Barium and lead
(c) Copper and mercury
(d) Platinum and gold
(vi) Dissimilar Elements were Placed in the Same Group
- Example: Silver and gold were placed in the same group while there is little similarity in physical and chemical properties.
(vii) Cause of Periodicity
- Mendeleev did not explain the cause of periodicity in the physical and chemical properties of the elements.
(viii) Metals have not been Separated from Non-Metals
(ix) Position for Elements of Group
- There is no proper position for the elements of the group.
(x) Consisting of Elements in Three Triads
- These elements are placed outside the main structure of the periodic table.
|
87 videos|276 docs|72 tests
|
FAQs on Dobereiner's Triads, Octaves & Mendeleev's Periodic Table - Physical Science for High School - Grade 9
| 1. What is Dobereiner's Triads Rule? |  |
| 2. What is Newlands Law of Octave? |  |
| 3. How is Mendeleev's Periodic Table different from Dobereiner's Triads and Newlands Law of Octave? |  |
| 4. Who is the founder of the modern periodic table? |  |
| 5. Why was Newlands Law of Octave criticized? |  |
















