Grade 11 Exam > Grade 11 Notes > Chemistry for Grade 11 (IGCSE) > Enthalpy Change in Bond Breaking and Bond Making
Enthalpy Change in Bond Breaking and Bond Making | Chemistry for Grade 11 (IGCSE) PDF Download
| Table of contents |

|
| Bond Breaking & Bond Forming |

|
| Exothermic Reactions |

|
| Endothermic Reactions |

|
| Bond Energy Calculations |

|
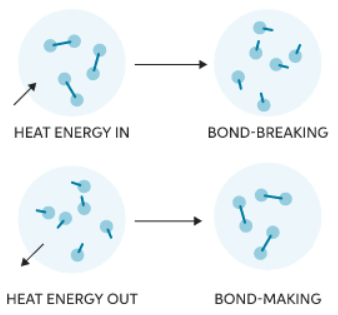
Bond Breaking & Bond Forming
- Whether a reaction is endothermic or exothermic depends on the energy required to break existing bonds and the energy released during new bond formation.
- Bond breaking involves absorbing energy from the surroundings to break chemical bonds, making it an endothermic process.
- For example, breaking the bonds in water (H2O) to form hydrogen (H2) and oxygen (O2) requires energy input.
- On the other hand, bond making releases energy to the surroundings during bond formation, classifying it as an exothermic process.
- When hydrogen (H2) and oxygen (O2) combine to form water (H2O), energy is released.
Exothermic Reactions
- If a reaction releases more energy than it absorbs, it is termed exothermic.
- Exothermic reactions involve more energy being released when new bonds are formed compared to the energy required to break the bonds in the reactants.
- The change in energy is negative since the products possess less energy than the reactants.
- Consequently, an exothermic reaction is characterized by a negative ΔH value.
- Making new chemical bonds releases energy, which is emitted as heat into the surroundings:

Question for Enthalpy Change in Bond Breaking and Bond MakingTry yourself:What type of reaction is characterized by a negative ΔH value?
View Solution
Endothermic Reactions
- If a reaction absorbs more energy to break bonds than it releases to form new bonds, it is termed endothermic.
- Endothermic reactions involve the products containing more energy than the reactants.
- The symbol ΔH (delta H) represents the change in heat energy, where H signifies enthalpy, measuring the total heat of a chemical reaction.
- Therefore, an endothermic reaction is identified by a positive ΔH value, evident in energy level diagrams and calculations.
- Breaking chemical bonds requires energy which is taken in from the surroundings in the form of heat:

Bond Energy Calculations
Energy of reaction calculations
- Every chemical bond possesses a distinct bond energy.
- This energy represents the amount needed to break the bond or released upon its formation.
- Calculating heat release or absorption in a reaction involves utilizing these bond energies.
- Knowledge of the bonds in both reactants and products is essential for this calculation.
Method
- If an equation is not provided, create a balanced one.
- Optionally, illustrate the displayed formula to discern bond type and quantity.
- Sum the bond energies across all bonds in the reactants, representing the "energy in" component.
- Sum the bond energies across all bonds in the products, representing the "energy out" component.
- Calculate the enthalpy change using the formula: ΔH = Energy absorbed - Energy released.
Question for Enthalpy Change in Bond Breaking and Bond MakingTry yourself:What is an endothermic reaction?
View Solution
The document Enthalpy Change in Bond Breaking and Bond Making | Chemistry for Grade 11 (IGCSE) is a part of the Grade 11 Course Chemistry for Grade 11 (IGCSE).
All you need of Grade 11 at this link: Grade 11
|
103 docs|53 tests
|
FAQs on Enthalpy Change in Bond Breaking and Bond Making - Chemistry for Grade 11 (IGCSE)
| 1. What is the difference between bond breaking and bond forming in chemical reactions? |  |
Ans. Bond breaking involves the energy needed to break existing chemical bonds, while bond forming involves the energy released when new chemical bonds are formed.
| 2. How do exothermic reactions differ from endothermic reactions in terms of bond energy? |  |
Ans. Exothermic reactions release energy to the surroundings, while endothermic reactions absorb energy from the surroundings. Exothermic reactions have a lower enthalpy of reaction compared to endothermic reactions.
| 3. How are bond energy calculations used to determine the overall enthalpy change in bond breaking and bond making during a chemical reaction? |  |
Ans. Bond energy calculations involve determining the energy required to break bonds in reactant molecules and the energy released when new bonds are formed in product molecules. The difference between these two values gives the overall enthalpy change in the reaction.
| 4. Why are bond energy calculations important in understanding the energy changes in chemical reactions? |  |
Ans. Bond energy calculations provide valuable insights into the energy changes that occur during chemical reactions, helping chemists predict reaction outcomes, design new reactions, and optimize reaction conditions.
| 5. How can enthalpy change in bond breaking and bond making be experimentally determined in the laboratory? |  |
Ans. Enthalpy change in bond breaking and bond making can be determined experimentally using calorimetry techniques, where the heat released or absorbed during a reaction is measured and used to calculate the enthalpy change.

|
Explore Courses for Grade 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches