Grade 11 Exam > Grade 11 Notes > Chemistry for Grade 11 (IGCSE) > Ethanoic Acids & Esterification Reactions
Ethanoic Acids & Esterification Reactions | Chemistry for Grade 11 (IGCSE) PDF Download
Formation of Ethanoic Acid
Making Carboxylic Acids
- Two methods for synthesizing carboxylic acids are oxidation through fermentation and using oxidizing agents.
- In fermentation, ethanol undergoes microbial oxidation, resulting in a weak solution of vinegar (ethanoic acid). This process occurs naturally when a bottle of wine is exposed to air, as bacteria (acetobacter) in the air oxidize ethanol with atmospheric oxygen:
C2H5OH (aq) + O2 (g) → CH3COOH (aq) + H2O (l) - The vinegary taste in wine left open for several days is due to the presence of ethanoic acid.
- Alternatively, carboxylic acids can be synthesized using oxidizing agents like potassium manganate(VII). This involves heating ethanol with acidified potassium manganate(VII) in the presence of an acid. The reaction is conducted under reflux, which involves heating the mixture in a vessel equipped with a condenser to prevent the volatile alcohol from escaping:
CH3CH2OH (aq) + 2[O] → CH3COOH (aq) + H2O (l) - During this process, the solution changes from purple to colorless. The oxidizing agent is represented by the symbol for oxygen in square brackets.
- Diagram showing the experimental setup for the oxidation with KMnO4 using reflux apparatus:

Esterification
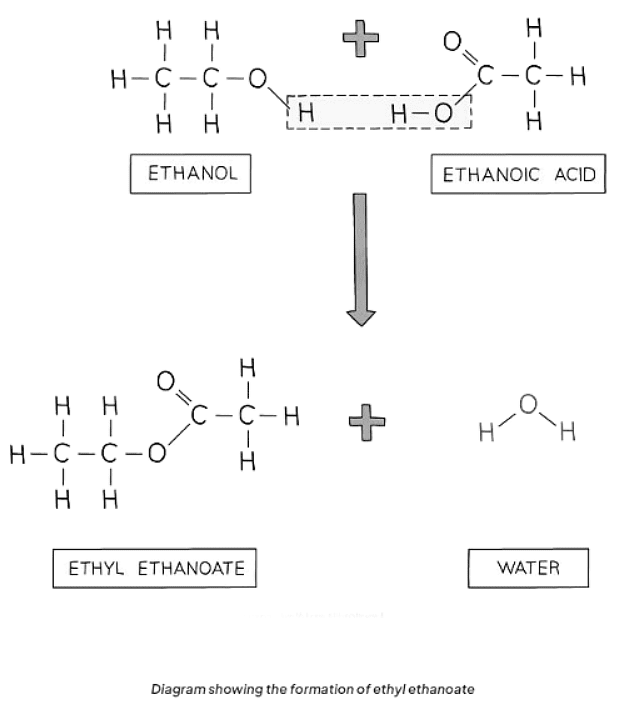
- Alcohols and carboxylic acids undergo esterification reactions to produce esters. Esters are characterized by the functional group R-COO-R and are commonly sweet-smelling oily liquids employed in food flavorings and perfumes.
- For instance, ethanoic acid reacts with ethanol in the presence of concentrated sulfuric acid (acting as a catalyst) to yield ethyl ethanoate:
CH3COOH (aq) + C2H5OH (aq) ⇌ CH3COOC2H5 (aq) + H2O (l)
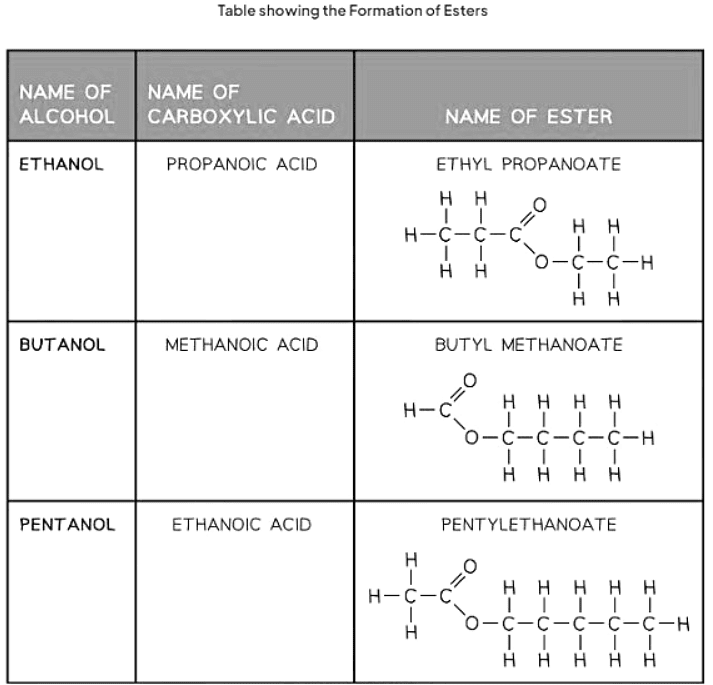
Naming Esters
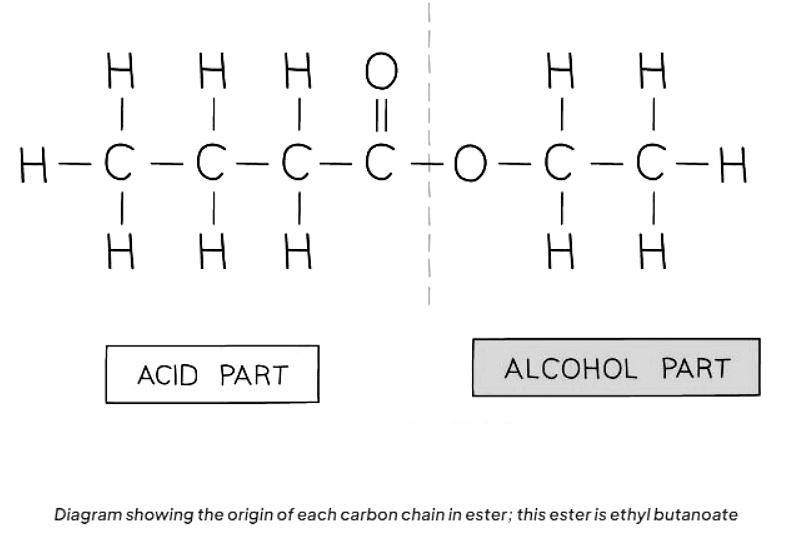
- Esters are derived from the reaction between an alcohol and a carboxylic acid. The naming convention for esters involves indicating the length of the carbon chain in both the alcohol and the carboxylic acid.
- The first part of the ester's name signifies the carbon chain length in the alcohol, ending with "-yl." Meanwhile, the second part indicates the carbon chain length in the carboxylic acid, ending with "-oate."
- For example, if pentanol and butanoic acid react, the resulting ester is named pentyl butanoate.
Question for Ethanoic Acids & Esterification ReactionsTry yourself: Which of the following methods can be used to synthesize carboxylic acids?View Solution
The document Ethanoic Acids & Esterification Reactions | Chemistry for Grade 11 (IGCSE) is a part of the Grade 11 Course Chemistry for Grade 11 (IGCSE).
All you need of Grade 11 at this link: Grade 11
|
103 docs|53 tests
|
FAQs on Ethanoic Acids & Esterification Reactions - Chemistry for Grade 11 (IGCSE)
| 1. What is the process of esterification? |  |
Ans. Esterification is a chemical reaction between an alcohol and a carboxylic acid to form an ester and water.
| 2. How is ethanoic acid formed through esterification? |  |
Ans. Ethanoic acid can be formed through the esterification reaction between ethanol (alcohol) and acetic acid (carboxylic acid), producing ethyl acetate (ester) and water.
| 3. What are some common uses of ethanoic acid? |  |
Ans. Ethanoic acid, also known as acetic acid, is commonly used in the production of vinegar, as a preservative in food, and in the manufacture of various chemicals.
| 4. How does esterification differ from other chemical reactions? |  |
Ans. Esterification is a specific type of chemical reaction that involves the formation of an ester compound through the combination of an alcohol and a carboxylic acid, while other reactions may involve different reactants and products.
| 5. Can esterification reactions be reversible? |  |
Ans. Yes, esterification reactions can be reversible, meaning that under certain conditions, esters can be hydrolyzed back into the original alcohol and carboxylic acid components.

|
Explore Courses for Grade 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches