GATE Chemistry Syllabus 2024 | GATE Chemistry Mock Test Series PDF Download

GATE Chemistry Syllabus 2024: Important Information
GATE has recently disclosed the dates, exam pattern, and the revised GATE Chemistry Syllabus for 2024. For aspirants gearing up for the GATE CY Exam, we have simplified the process of grasping all critical points.
Who is Eligible for GATE 2024?
For GATE 2024, eligibility criteria are as follows:
- Education Qualification: Candidates must possess a graduate degree or be in the final year of an undergraduate program in Arts, Commerce, Engineering, Science, Architecture, or Technology from a recognized institution in India.
- Age Limit: There is no age restriction set by the exam authorities for GATE 2024.
GATE CY Exam 2024: GATE CY Syllabus and Exam Pattern
In order to understand the GATE Chemistry (CY) syllabus and exam pattern for 2024, refer to the following points:
- Review the GATE Chemistry Syllabus and Exam Pattern to gain a comprehensive understanding of the exam structure.
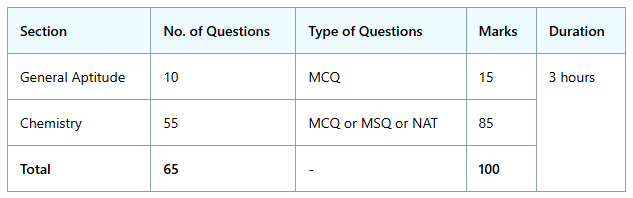
- The GATE CY paper comprises two sections: General Aptitude (GA) and Chemistry (CY).
- The exam is conducted in a Computer Based Test format with a total duration of 3 hours.
- The total marks for the GATE CY paper amount to 100, with 15 marks allocated for General Aptitude and 85 marks for Chemistry.
- General Aptitude includes Multiple-choice Questions (MCQs) with each question carrying either 1 or 2 marks.
- The Chemistry section encompasses MCQs, Numerical Answer Type (NAT) questions, and Multiple-Select Questions (MSQs), each question carrying one or two marks.
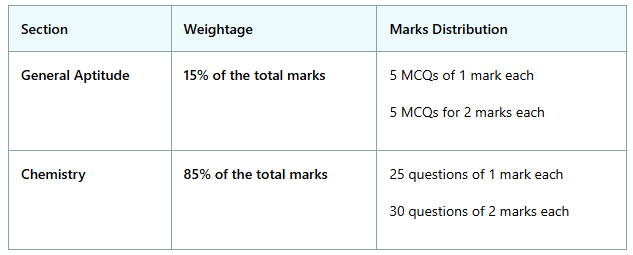
- General Aptitude holds a weightage of 15% of the total marks, while Chemistry constitutes 85% of the total marks.
Below is a tabular representation of the GATE CY Exam Pattern 2024:
Marks weightage for the GATE CY Exam is as follows:
GATE Chemistry Syllabus 2024
GATE CY Syllabus contains two parts – General Aptitude and Chemistry. General Aptitude consists of verbal, analytical, spatial, and numerical aptitude. The GATE Chemistry Syllabus is broadly divided into – physical chemistry, inorganic chemistry, and organic chemistry.
Subjects GATE CY Syllabus 2024
- Chemistry
- General Aptitude
Chemistry syllabus is divided into three main branches: Physical Chemistry, Inorganic Chemistry, and Organic Chemistry. Let's delve into the specifics of each branch:
These topics form the core of the GATE Chemistry Syllabus 2024. Understanding these concepts thoroughly will be crucial for excelling in the examination.
Gate General Aptitude Syllabus 2024
| Subjects | Topics |
|---|---|
| Verbal Ability | Basic English grammar includes parts of speech, verb-noun agreement, tenses, articles, conjunctions, adjectives, and prepositions. Basic vocabulary covers idioms, words, and contextual phrases for effective communication. Narrative sequencing is important for understanding stories and texts in a logical order. Reading and comprehension skills are essential for interpreting and analyzing written material. |
| Quantitative Ability | Data representation involves interpreting information from data graphs like bar graphs, pie charts, and tables. Topics include mensuration, geometry, probability, statistics, permutations, combinations, percentage, ratios, power, series, exponents, and logarithms. |
| Analytical Ability | Analogy questions test your ability to identify relationships between different elements. Number relations and reasoning involve understanding numerical relationships and patterns. Logic includes deduction and induction for drawing conclusions based on given premises. |
| Spatial Aptitude | Tasks may involve paper folding, cutting, and recognizing patterns in 2-D and 3-D shapes. Transformation of shapes includes rotation, mirroring, scaling, assembling, translation, and grouping. |
GATE Chemistry Syllabus 2024: Physical Chemistry
| Subjects | Topics |
|---|---|
| Group Theory | Topics cover point groups, character tables, hybrid orbital construction, symmetry elements, and symmetry operations. Learn about internal coordinates, vibrational modes, and symmetry-adapted linear combinations of atomic orbitals (LCAO-MO). |
| Equilibrium | Thermochemistry and thermodynamics laws, functions, and their relationships are crucial for understanding energy transformations. Topics include mixing thermodynamics, statistical thermodynamics, chemical potential, equilibrium constants, and phase rule. |
| Structure | Quantum mechanics postulates and molecular structure are fundamental for understanding chemical bonding. Study topics such as valence bond theory, LCAO-MO theory, multi-electron atoms, and molecular orbital theory. |
| Spectroscopy | Explore diatomic and polyatomic molecular spectroscopy, including vibrational, rotational, and electronic spectroscopy. Understand nuclear magnetic resonance principles, term symbols, and selection rules. |
| Kinetics | Learn about reaction kinetics, photochemical processes, polymerization kinetics, and effects of kinetic isotopes. Topics include elementary reactions, catalysis, enzyme catalysis, and potential energy surfaces. |
| Surfaces and Interfaces | Understand Langmuir, Freundlich, and BET isotherms, surface tension, and catalysis mechanisms. Explore colloids, micelles, and physical chemistry of macromolecules. |
GATE Chemistry Syllabus 2024: Organic Chemistry
Subjects | Topics |
|---|---|
| Organic Synthesis | Atom economy and Green Chemistry; Protection and deprotection of functional groups; Concepts of multistep synthesis; Selectivity in organic synthesis (chemoselectivity, regioselectivity and stereoselectivity); Concepts of asymmetric synthesis; Uses of Li, Zn, Cu, B, Mg, Sn, P, Si, and S based reagents in organic synthesis; Synthesis, reactions, mechanisms and selectivity (alkenes, alkynes, arenes, alcohols, phenols, aldehydes, ketones, carboxylic acids, esters, nitriles, halides, nitro compounds, amines and amides); Umpolung reactivity (formyl and acyl anion equivalents); Stereoselective addition to C=O groups (Cram, Prelog and Felkin-Anh models); Carbon-carbon bond formation through coupling reactions (Hiyama, McMurry, Tsuji-Trost, Kumada, Sonogoshira, Suzuki, Negishi, Stille, olefin metathesis and Heck); Carbon-carbon and carbon-heteroatom bond forming reactions through enolates (including boron enolates), enamines and silyl enol ethers. |
| Reaction Mechanism | Basic mechanistic concepts (kinetic vs thermodynamic control, postulate of Hammond and principle of Curtin-Hammett); Nucleo- & electrophilic substitution reactions; Linear free-energy relationship (Hammett & Taft equations); Reactive intermediates (carbenes, nitrenes, carbanions, carbocations, arynes & free radicals); Addition reactions to carbon-carbon and carbon-heteroatom (N and O) multiple bonds; Elimination reactions; Methods to determine reaction mechanisms with the help of kinetics, products identification, intermediates & isotopic labelling; Molecular rearrangements. |
| Stereochemistry | Homo-, enantio- and diastereotopic atoms, faces & groups; Geometrical & optical isomerism; acyclic and cyclic compounds’ conformational analysis; Stereoselective and stereospecific synthesis; Relative stereochemistry in compounds with more than one stereo genic centre; Chirality & symmetry of organic molecules in relation to chiral centres & absolute configurations; Configurational and conformational effects, atropisomerism, & neighboring group participation on reactivity and selectivity or specificity. |
| Biomolecules | Chemical structural features of lipids, steroids, proteins, carotenoids, nucleic acids, alkaloids, and terpenoids; Mono-saccharides & di-saccharides (structure, reaction, & properties); chemical synthesis and structure of peptides; physicochemical properties of amino acids. |
| Pericyclic Reactions & Photochemistry | Norrish type-I and II cleavage reaction; Photochemistry of alkenes, arenes and carbonyl compounds; Electrocyclic, cycloaddition & sigmatropic reactions; Orbital correlations (FMO & PMO treatments, Woodward-Hoffmann rule); Di-π-methane rearrangement; Photooxidation & photoreduction; Barton-McCombie reaction. |
| Heterocyclic Compounds | Furan, quinoline, pyridine, thiophene, isoquinoline, indole, and pyrrole (Structure, preparation, properties and reactions). |
| Experimental Techniques in Organic Chemistry | Chromatographic techniques application (thin-layer, column, HPLC & GC); IR, UV-visible, NMR & Mass spectrometry application in determining structure of organic molecules; Polarimetry. |
GATE Chemistry Syllabus: Inorganic Chemistry
Subjects | Topics |
|---|---|
| Main Group Elements | Industrial synthesis of main group elements compounds; Shapes & Reactivity of oxides, sulfides, oxoacids, hydrides, nitrides, halides, boron nitride, phosphazenes, boranes, silicones, borazines, carboranes, and silicates; Noble gases, pseudo halogens, and interhalogen compounds chemistry; Acid-base concepts and principles; Sulphur, Carbon, and phosphorus allotropes. |
| Transition Elements | Energy level diagrams in CFSE, CFT, various crystal fields, and Jahn-Teller distortion; Coordination chemistry (structure & isomerism); Transition metal complexes and its magnetic properties; Bonding theories (VBT, CFT, & MOT); Ray-Dutt & Bailar twists; Electronic spectra of transition metal complexes; Metal-metal multiple bond; Reaction mechanisms (kinetic & thermodynamic stability, substitution & redox reactions). |
| Lanthanides & Actinides | Its recovery, spectra, periodic properties, & magnetic properties. |
| Organometallics | Organometallic reaction types, fluxionality in complexes of organometallics; metal-alkyl, metal-carbonyl, metal-olefin and metal-carbene complexes and metallocene; 18-Electron rule; Heterogeneous catalysis - Ziegler-Natta polymerization, Fischer-Tropsch reaction; Homogeneous catalysis - hydroformylation, metathesis and olefin oxidation, hydrogenation, and acetic acid synthesis. |
| Solids | Bragg’s law; band theory; Crystal systems and lattices; Crystal packing; ionic crystals; Miller planes; crystal defects; spinels, metals and semiconductors; structures of AX, AX2, ABX3 type compounds. |
| Instrumental Methods of Analysis | Electro- and thermoanalytical methods; UV-visible; X-ray crystallography; fluorescence and FTIR spectrophotometry; Chromatography including GC and HPLC; NMR and ESR, atomic absorption, and Mössbauer (Fe and Sn) spectroscopy; mass spectrometry. |
| Bioinorganic Chemistry | Oxygen binding; Ion (Na+ and K+) transport; nitrogen fixation; electron transfer reactions; transport and utilization; metalloenzymes containing Mg, Mo, Fe, Co, Cu, and Zn. |
| Radioactivity | Radioactivity detection; fission & fusion processes; Half-life of radioactive elements; Decay processes. |
Basic Tips for Cracking GATE Chemistry Paper 2024
- Make completing the GATE CY Syllabus your top aim.
- Focus on time management by solving as many mock tests and previous year question papers.
- Revision is the key to successful preparation. Draw your prep plan accordingly. Make sure to finish the GATE Syllabus at least a month prior to the main examination.
- While preparing, make sure that you are not carried away preparing for general aptitude that you compromise with your main paper.
- If you are preparing for the exam then you can download GATE Chemistry Exam Previous Year Papers here!
Knowing the syllabus in detail will also help you understand how to prepare for the exam and where to start from.
|
18 docs|37 tests
|
FAQs on GATE Chemistry Syllabus 2024 - GATE Chemistry Mock Test Series
| 1. Who is eligible to appear for GATE Chemistry Exam 2024? |  |
| 2. What is the exam pattern for GATE CY 2024? |  |
| 3. What are the important topics covered in the GATE Chemistry Syllabus 2024? |  |
| 4. How can one effectively prepare for the GATE Chemistry Paper 2024? |  |
| 5. What are some basic tips for scoring well in the GATE Chemistry Exam 2024? |  |

|
Explore Courses for GATE Chemistry exam
|

|


















