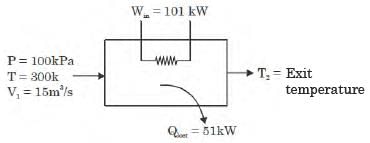
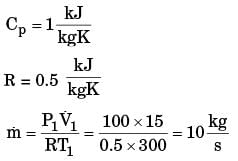
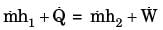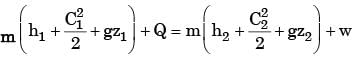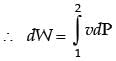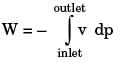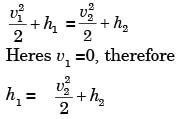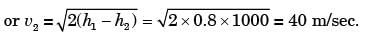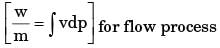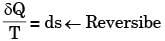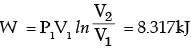GATE Past Year Questions: First Law Of Thermodynamics, Heat, Work And Energy Interactions | Thermodynamics - Mechanical Engineering PDF Download
Q1: A heat pump (H.P.) is driven by the work output a heat engine (H.E.) as shown in the figure. The heat engine extracts 150kJ of heat from the source at 1000k. The heat pump absorbs heat from the ambient at 280K and delivers heat to the room which is maintained at 300k Considering the combined system to be ideal, the total amount of heat delivered to the room together by the heat engine and heat pump is ____ kJ (answer in integer). (2024)
(a) 1254
(b) 2358
(c) 1620
(d) 1456
Ans:(d)
Sol:

or,

So, net heat delivered to room
Q2: A piston-cylinder arrangement shown in the figure has a stop located 2 m above the base. The cylinder initially contains air at
140 kPa and 350 o C and the piston is resting in equilibrium at a position which is 1m above the stops. The system is now cooled to the ambient temperature of 25oC Consider air to be an ideal gas with a value of gas constant R=0.287 kJ (kg.K) The absolute value of specific work done during the process is _______ kJ/kg (rounded off to 1 decimal place). (2024)
(a) 59.6
(b) 63.2
(c) 47.5
(d) 88.6
Ans:(a)
Sol:


For constant pressure process
[as the stopper, length is 2 m area is constant]
Q3: Consider a fully adiabatic piston-cylinder arrangement as shown in the figure. The piston is massless and cross-sectional area of the cylinder is A. The fluid inside the cylinder is air (considered as a perfect gas), with 𝛾 being the ratio of the specific heat at constant pressure to the specific heat at constant volume for air. The piston is initially located at a position L 1 The initial pressure of the air inside the cylinder is P1 ≫ P0 , where P0 is the atmospheric pressure. The stop S1 is instantaneously removed and the piston moves to the position L2 where the equilibrium pressure of air inside the cylinder isP2 ≫ P0 (2023)
What is the work done by the piston on the atmosphere during this process?
(a) 0
(b) P0 A(L2 - L1 )
(c) 
(d) 
Ans :(b)
Sol: 
Initial volume V 1 = L1 × A
Final volume 𝑉 2 = 𝐿 2 × 𝐴
Work done by atmospheric air=

Q4: A heat engine extracts heat (QH) from a thermal reservoir at a temperature of 1000K and rejects heat(QL)
to a thermal reservoir at a temperature of 100K, while producing work (W). Which one of the combinations of
(QH ,QL,W) given is allowed? (2023)
(a) QH=2000J,QL=500J,W=1000J
(b) 𝑄𝐻=2000𝐽,𝑄𝐿=750𝐽,𝑊=1250𝐽
(c) 𝑄𝐻=6000𝐽,𝑄𝐿=500𝐽,𝑊=5500𝐽
(d) QH=6000J,QL=600J,W=5500J
Ans:(b)
Sol: For a reversible engine, the rate of heat rejection is minimum.
For process to be feasible
for option 
So cyclic process is possible.
Q5: Consider 1 kg of an ideal gas at 1 bar and 300 K contained in a rigid and perfectly insulated container. The specific heat of the gas at constant volume cv is equal to 750 Jkg -1K-1 . A stirrer performs 255 kj of work on the gas. Assume that the container does not participate in the thermodynamic interaction. The final pressure of the gas will be ______ bar (in integer). (2022Set 2)
(a) 1
(b) 2
(c) 3
(d) 4
Ans: (b)
Sol: m=1kg,P1=1bar,T1=300K
V=Constant
Wexpansion =0
Using Ist law of thermodynamics
Q-W =dU =mcv (T2 -T1)
0-(-255) =1×0.75(T2−300)
T2 =600k
Q6: A polytropic process is carried out from an initial pressure of 110 kPa and volume of 5m3 to a final volume of 2.5m3. The polytropic index is given by n = 1.2. The absolute value of the work done during the process is _______ kJ (round off to 2 decimal places). (2022 Set 1)
(a) 408.92
(b) 215.58
(c) 852.36
(d) 789. 14
Ans(a)
Sol: Polytropic process]



Q7: In a steam power plant, superheated steam at 10 MPa and 5000C. is expanded isentropically in a turbine until it becomes a saturated vapour. It is then reheated at constant pressure to 5000C. The steam is next expanded isentropically in another turbine until it reaches the condenser pressure of 20 kPa. Relevant properties of steam are given in the following two tables. The work done by both the turbines together is ______ kJ/kg (roundoff to the nearest integer). (2020Set 2) 

(a) 1513
(b) 1245
(c) 832
(d) 1825
Ans: (a)
Sol:
Given data: 


Q8: Moist air at 105 kPa, 300 C and 80% relative humidity flows over a cooling coil in an insulated air-conditioning duct. Saturated air exits the duct at 100 kPa and 150C The saturation pressure of water at 300 C and150C are 4.24 kPa and 1.7 kPa respectively. Molecular weight of water is 18 g/mol and that of air is28.94 g/mol .The mass of water condensing out from the duct is ______ g/kg of dry air (round off to 2 decimal places). (2020 Set2)
(a) 8.21
(b) 15.24
(c) 10.01
(d) 12.24
Ans(c)
Sol: 






Q9: Air is contained in a frictionless piston-cylinder arrangement as shown in the figure.
The atmospheric pressure is 100 kPa and the initial pressure of air in the cylinder is 105 kPa. The area of piston is 300cm2. Heat is now added and the piston moves slowly from its initial position until it reaches the stops The spring constant of the linear spring is 12.5 N/mm Considering the air inside the cylinder as the system, the work interaction is ________ J. (round off to the nearest integer)
(2020Set2)
(a) 1
(b) 544
(c) 254
(d) 623
Ans: (b)
Sol: 
1-2 constant pressure




Alternate Solution:
Total work = Workdone because of 105 kPa pressure + Workdone against spring which is equal to energy stored in spring

Q10: One kg of air, initially at a temperature of 127OC , expands reversibly at a constant pressure until the volume is doubled. If the gas constant of air 287 J/kg .K the magnitude of work transfer is __________ kJ (round off to 2 decimal (2020Set1)
(a) 156.6
(b) 114.8
(c) 89.8
(d) 124.4
Ans: (b)
Sol:


[2019 , Set 1]
[2017, Set-2]
[2009]
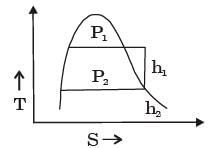
[2008] where v is the specific volume and p is the pressure, The expression for w given above
where v is the specific volume and p is the pressure, The expression for w given above
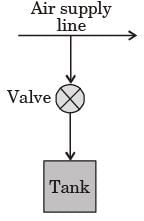
A valve connected with the supply line is opened and the tank is charged with air until the final pressure inside the tank reaches 1 MPa. The final temperature inside the tank [2008]
The tank is connected with a supply line through which air (assumed to be ideal gas with constant specific heats) passes at 1 MPa, 350°C.
[2001]
[2000]
[2000]
[1996]
[2011]
[2008]
[2007]
[2006]
[2006]
[2003]
[1993]
[2016 , Set-2]
[2015, Set-1]
[2009]
[2008]
|
29 videos|65 docs|36 tests
|
FAQs on GATE Past Year Questions: First Law Of Thermodynamics, Heat, Work And Energy Interactions - Thermodynamics - Mechanical Engineering
| 1. What is the first law of thermodynamics? |  |
| 2. How is heat related to the first law of thermodynamics? |  |
| 3. Can you explain the concept of work in the context of the first law of thermodynamics? |  |
| 4. How do heat and work interactions play a role in the first law of thermodynamics? |  |
| 5. What are some real-world applications of the first law of thermodynamics? |  |

|
Explore Courses for Mechanical Engineering exam
|

|