Group 13 Elements: Boron Family | Inorganic Chemistry PDF Download
| Table of contents |

|
| p-Block Elements |

|
| Inert Pair Effect |

|
| Group 13 Elements (Boron Family) |

|
| Atomic And Physical Properties |

|
| Properties of Boron |

|
| Compounds Of Boron |

|
| Aluminum (Al) |

|
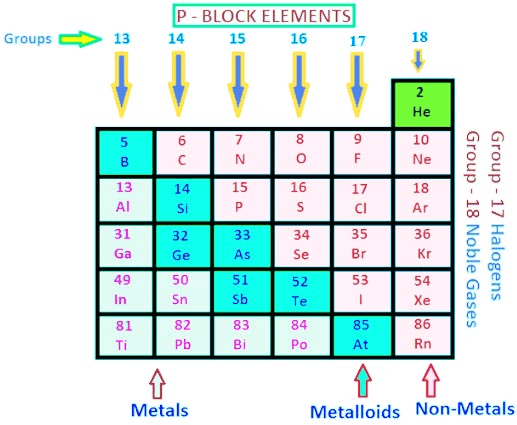
p-Block Elements
The elements in which the last electron enters the outermost p orbital are called p-block elements.
- As the maximum number of electrons that can be accommodated in a set of p-orbitals is six, therefore there are six groups of p-blocks in the periodic table.
- The elements from the group 13 to 18 in the periodic table are p-Block elements
Inert Pair Effect
Inert Pair Effect is a decrease in stability of the maximum oxidation state and an increase in the stability of the (maximum-2) state on descending the group.
- The inert pair effect shows itself increasingly in the heavier members of the group.
- Ge(+II) is a strong reducing agent whereas Ge(+IV) is stable Sn(+II) exists as simple ions which are strongly reducing but Sn(+IV) is covalent and stable.
- Pb(+II) is ionic, stable, and more common than Pb(+IV), which is oxidizing.
- The lower valencies are more ionic because the radius of M2+ is greater than that of M4+ and according to Fajans rules, the smaller the ion the greater the tendency to covalency.
Group 13 Elements (Boron Family)
The Elements are B( Nonmetal), Al, Ga, In, Tl (metals)
General electronic configuration [Noble gas] ns2 np1
Atomic And Physical Properties
(1) Atomic and Ionic radii
Atomic radii: B > Ga < Al < In < Tl
(2) Ionization Enthalpies.
B > Tl > Ga > Al > In
(3) Melting and Boiling points
M.P. B > Al > Tl > In > Ga
B.P. B > Al > Ga > In > Tl
(4) Electropositive Character
Due to high IE they are less electropositive on moving down the group metallic character increases due to decreases in IE [\B is nonmetals and other elements are metals]
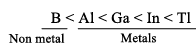
Properties of Boron
It exists in five forms four of which are crystalline and one is amorphous. All crystalline forms are very hard made up of clusters of B12 units. All crystalline forms are black in appearance and chemically inert. Melting points are around 2300°C. But amorphous form is brown and chemically active.
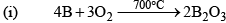

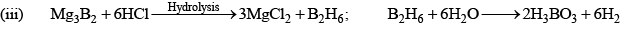
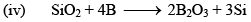
Compounds Of Boron
Boron Trioxide(B2O3)
Preparation
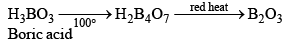
Properties
It is a weakly acidic oxide and reacts with alkalies or bases to form borates
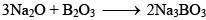 ( Sodium orthoborate), It reacts with water slowly to form orthoboric acid. When heated with transition metal salts if forms coloured compounds.
( Sodium orthoborate), It reacts with water slowly to form orthoboric acid. When heated with transition metal salts if forms coloured compounds.
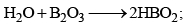
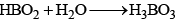
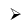 The oxyacids of boron are:
The oxyacids of boron are:
(a) Orthoboric acid (b) Metaboric acid (HBO2) (c) Tetraboric acid (H2B4O7) (d) Pyroboric acid (H4B4O8)
Orthoboric Acid
Orthoboric acid H3BO3 behaves as a weak monobasic acid it does not donate protons but rather accepts OH–. It is therefore a Lewis acid B(OH)3. Due to sp2 hybridization of boron, boric acid is a planar molecule and due to H-bonding between different molecules boric acid has a layer structure.
Preparation
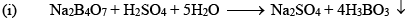
H3BO3 is soluble in water and behaves as weak monobasic acid. It does not donate protons like most acids, but rather it accepts OH–. It is, therefore, a Lewis acid (B(OH)3)
Properties
- It is a weak monobasic Lewis acid and in aqueous solution, the boron atom completes its octet by removing. OH– from water molecules.
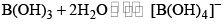
- Since B(OH)3 only partially reacts with water to form H3O+ and [B(OH)4]– it behaves as a weak acid.
- Thus it cannot be titrated satisfactorily with NaOH as a sharp endpoint is not obtained. If certain polyhydroxy compounds such as glycerol, mannitol, or sugar are added to the titration mixture then B(OH)3 behaves as a strong monobasic acid.
- And hence can now be titrated with NaOH and endpoint is detected using phenolphthalein as indicator.
- The added compound must be cis diol to enhance the acidic properties in this way the cis-diol forms very stable complexes with [B(OH) 4]– formed in forward direction above, thus effectively removing it from solution.
- Hence reaction proceeds in forward direction (Le-Chatelier principle)
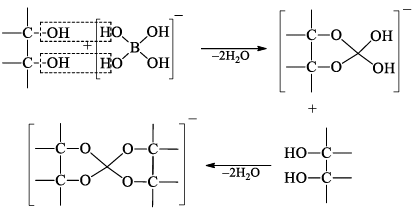
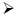 Heating of boric acid:
Heating of boric acid:
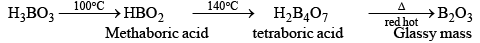
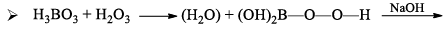

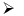 When boric acid is heated with ethyl alcohol, the evolved gas vapours of ethylborate is burned forming a green-edged flame.
When boric acid is heated with ethyl alcohol, the evolved gas vapours of ethylborate is burned forming a green-edged flame.
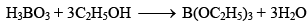
Borax (Na2B4O7.10H2O)
Preparation of Borax:
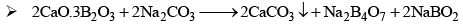

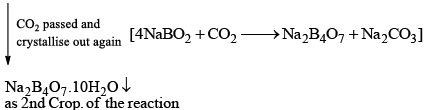
Properties
- Its aqueous solution is alkaline because of its hydrolysis to weak acid H3BO3 and strong alkali NaOH
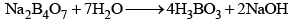
(ii) When borax powder is heated, it first swells due to loss of water in the form of steam but at 740°C it becomes converted into colourless transparent borax bead.
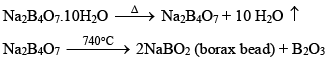
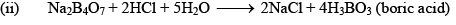
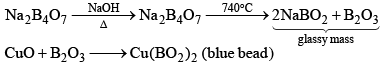
Boron Hydrides
Binary compounds of B with H are called boron hydrides of boranes. These compounds form following two types of series.
(Nido) BnHn+4 → B2H6, B5H9, B6H10, B10H14
(Arachno) BnHn+6 → B4H10, B5H11, B6H12, B9H15
Diborane (B2H6)
Structure of Diborane
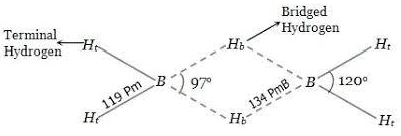
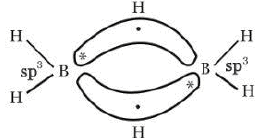
Preparation
(i) 4BCl3 + 3LiAlH4 → 2B2H6 + 3LiCl + 3AlCl3
Properties:
 B2H6 + 3O2 →B2O3 + 2H2O; ΔH = -2137.7 kJ mol-1
B2H6 + 3O2 →B2O3 + 2H2O; ΔH = -2137.7 kJ mol-1
 Pyrolysis of B2H6 in sealed vessels at temperatures above 375 K is an exceedingly complex process producing a mixture of various boranes e.g. B4H10, B6H12 and B10H14
Pyrolysis of B2H6 in sealed vessels at temperatures above 375 K is an exceedingly complex process producing a mixture of various boranes e.g. B4H10, B6H12 and B10H14
 B2H6 + 6MeOH → 2B(OMe)3 + 6H2
B2H6 + 6MeOH → 2B(OMe)3 + 6H2
 B2H6 + 2Me3N → 2Me3N – BH3
B2H6 + 2Me3N → 2Me3N – BH3
 B2H6 + 2Me3N →2Me3PBH3
B2H6 + 2Me3N →2Me3PBH3
 B2H6 + 2CO
B2H6 + 2CO 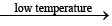 B2H62NH3
B2H62NH3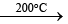 B3N3H6 (borazine)
B3N3H6 (borazine)
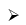 B2H6 + 2LiH → 2LiBH4
B2H6 + 2LiH → 2LiBH4
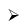 Carbaboranes are compounds of carbon, boron, and hydrogen in which carbon and born atoms occupy the vertices of a triangulated polyhedron. The compounds may be considered to have been derived from boranes by the replacement of BH– units by CH units. The carboranes and the boranes having the same number of electrons in their bonding, framework have similar skeletal structures. Three structurally different types of carboranes, namely, closo, nido and arachno are known at present.
Carbaboranes are compounds of carbon, boron, and hydrogen in which carbon and born atoms occupy the vertices of a triangulated polyhedron. The compounds may be considered to have been derived from boranes by the replacement of BH– units by CH units. The carboranes and the boranes having the same number of electrons in their bonding, framework have similar skeletal structures. Three structurally different types of carboranes, namely, closo, nido and arachno are known at present.
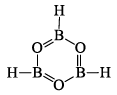
Borazine Or Borazole, (BH)3 or B3N3H6
This compound is isoelectronic with benzene and hence has been called Inorganic Benzene.
Preparation
Borazine can be prepared by the following methods.
(i) By Stock and Pohland’s method (1926): By the action of NH3 on diborane (B2H6). The adduct, B2H6. 2NH3 is first formed, which then gets decomposed by heating in a closed tube at 200°C.

(ii) By heating BCl3 with NH4Cl:
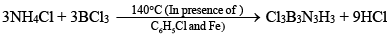

(iii) By heating mixture of LiBH4 and NH4Cl (Laboratory method):
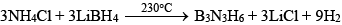
Chemical Properties
(i) Addition reactions:
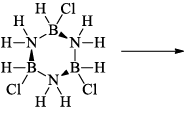
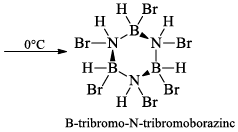
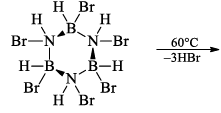
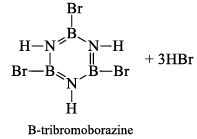
(a) One molecule of B3N3H6 add three molecules of HCl or HBr in the cold, without a catalyst. These molecules get attached with all the three B -atoms, of B3N3H6 molecule, since B -atom is more negative than N-atom in B—N or B=N bond and hydrogen chloride derivative (B3N3H9Cl) is obtained. This addition reaction is not shown by benzene.
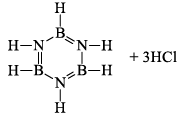
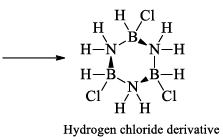
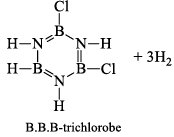
When this derivative is heated to 50 -100 °C, it looses, three H2 molecules, to give B, B, B -trichloroborazine, B3N3H3Cl3.
(b) 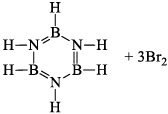
(ii) Hydrolysis:
(a) Borazine gets slowly hydrolyzed by water to produce boric acid [B(OH)3 or H3BO3], NH3, and H2. Hydrolysis is favoured by the increase in temperature.
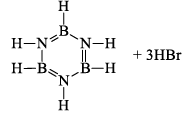
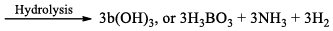
(b) It is reported that under proper conditions, borazine reacts with three molecules of water and gives B -trihydroxyl borazine, B3N3H3(OH)3 (substitution reaction).
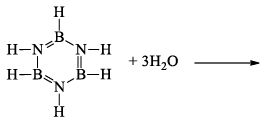
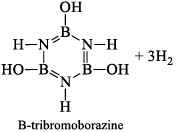
(iii) Pyrolysis: When borazine is pyrolyzed above 340°C, B6N6H10 and B5N5H8 are produced. These products are boron-nitrogen analogs of diphenyl and naphthalene respectively
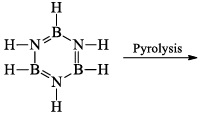
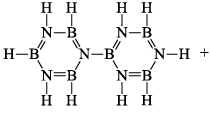

(iv) Hydrogenation: Hydrogenation of borazine produces polymeric materials of indefinite composition.
(v) Formation of adduct: Borazine forms an adduct with CH3OH. This adduct undergoes pyrolysis with the elimination of H2 and gives B-trimethoxy-borazine.
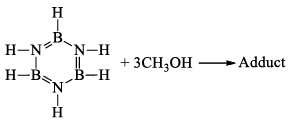
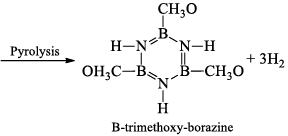
(vi) Reaction with aniline: Borazine undergoes a strongly exothermic reaction with aniline produce tri -aminoborine.
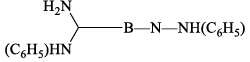
Structure of borazine molecule:
- In the structure of borazine, both B and N atoms are sp2 hybridized. Each N-atom has one lone pair of electrons, while each B-atom has an empty p-orbital. (B—N) π-bond in borazine is a dative bond, which arises from the sidewise overlap, between the filled p-orbitals of N-atom and empty p-orbitals of B-atom.
- Since borazine is isoelectronic with benzene, both the compounds have aromatic π-electron cloud (electrons in π-orbitals).
- Due to greater differences in the electronegativity values of B and N-atoms, the π-electron cloud in B3N3 ring of borazine molecule is partially delocalized, while in case of the benzene ring, the π-electron cloud is completely delocalized.
- In fact, complete delocalization of π-electron cloud in B3N3 ring in borazine molecule, cannot be expected, since N -π orbitals are of lower energy than the B -π orbitals.
- Molecular orbital calculations have indicated that π-electron drift from N to B is less than the σ-electron drift from B to N, due to greater electronegative of N-atom.
- In C6H6 molecule, C=C bonds are nonpolar while in the case of B3N3H6 molecule due to the difference in electronegativities between B and N atoms, B—N bond is polar.
- The ring structure of borazine molecule is the same as the layer lattice structure of boron nitride, (BN)n.
- It is due to the partial delocalisation of the π-electron cloud that π-bonding in B3N3 ring weakened. In addition, N-atom retains some of its basicity and the boron atom retains some of its acidity.
- Polar species like HCl, therefore, attack the double bond between N and B. Thus, borazine in contrast to C6H6, readily undergoes addition reactions.
- In these reactions, more electronegative atom (e.g., Cl in HCl molecule) is generally attached with B-atom, which is less electronegative then N in B—N bond.
- In borazine, B—N bond length is equal to 1.44 Å, which is between the calculated single B—N bond (=1.54 Å) and double bond, B=N(=1.36 Å) distances. The angles are equal to 120°. IN benzene C—C bond length is equal to 1.42 Å.
B-trimethyl borazine, [B(CH3)]3(NH)3
It is prepared by heating B(CH3)3 with NH3, at 320-340° atm for 2 hours.
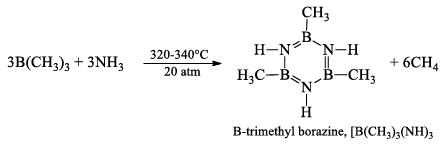
At 100°C, water replaces the NH groups by O-atoms and gives B-trimethyl boraxime, [B(CH3)]3O3.
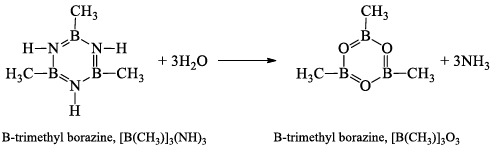
Boraxine,(BH)3O3
This compound is isoelectronic with borazine, B3N3H6(B3O3H3) = 3 × 3 + 3 × 6 + 3 × 1 = 30, B3N3H6 = 3 × 3 + 5 × 3 + 6 × 1 = 30. It is produced by the explosive oxidation of B2H6 or B5H9. This compound decomposes at room temperature to diborane (B2H6) and boron trioxide (B2O3).
2B3O3H3 → 2B2O3 + B2H6
Boraxine exhibits the aromatic properties of benzene. Boraxine is even less stable and presumably has less p-delocalization than borazine. In this molecule, B—O bond distance is equal to 1.38 Å. The characteristic Roman frequency of the ring is at 8.7 cm-1. B=O, double bond present in the structure is due to the donation of a lone pair of electrons from O-atom to boron atom. This results in the development of a formal negative charge on B-atom and equal formal positive charge on O-atom.
N-trimethyl borazine, (BH)3[N(CH3)]3
It is obtained in 90% yield by heating a mixture of B2H6 and NH2.(CH3) in the correct proportions at 180-200°C for 2 hours.
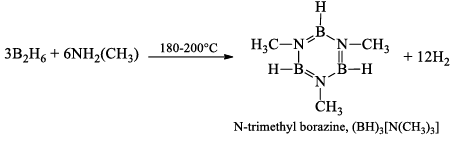
This compound can also be prepared by reducing Monomethyl ammonium chloride. (CH3)NH3Cl with lithium borohydride LiBH4.
3(CH3)NH3Cl + 3LiBH4 → (BH)3[N(CH3)]3 + 3LiCl + 9H2
Boron Halides
- The relative strength of Lewis acids of boron trihalides increases in the order BF3 < BCl3 < BBr3. This order of Lewis acid strength is just reversed of that expected on the basis of the electronegativity of the halogen. This is explained on the basis of overlapping of halogens sidewise with empty 2p-orbital of B forming pp-pp back bonding. The tendency of back bonding decreases as BF3 > BCl3 > BBr3 > Bl3 due to the difference in the energy states of the orbitals involved.
- Both boron and aluminum halides are Lewis acid but only aluminum halides exist as dimers whereas boron halides exist only as monomers. This is due to the reason that boron atom is so small that it cannot accommodate four large-sized halide ions around it.
- BF3 and AlCl3 are widely used as Lewis acid catalysts in Friedel -Crafts reactions and many industrial processes.
- Solution of AlEt3 and TiCl4 in hydrocarbon solvent reacts endothermically to form a brown solid. This is the important Ziegler Natta catalyst for polymerizing ethene to form polythene.
Aluminum (Al)
Properties
(i) Action of air: Dry air has no action of Al. But moist air forms a thin layer of Al2O3 on its surface and it loses its luster. At very high temperatures it burns to form Al2O3 and AlN.
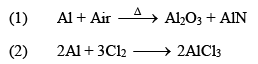
AlN reacts with hot water to form Al(OH)3 and NH3.
Compounds of Aluminium
(i) Aluminium Oxide (Al2O3) Alumina
Preparation
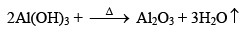
Properties
It is a white amorphous powder insoluble in water but soluble in acids (forming eg., AlCl3) as well as alkalies (forming NaAlO2). Thus amphoteric in nature. It is a polar covalent compound.
(ii) Aluminium Chloride (AlCl3.6H2O)
It is a colorless crystalline solid, soluble in water. It is anhydrous AlCl 3 is a deliquescent white solid.
Preparation
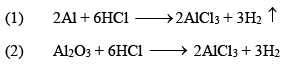
Properties
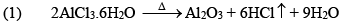
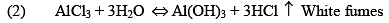
(iii) Al2Cl6, Al2Me6
Aluminum halides are dimers in the gas phase: aluminum chloride has the molecular formula Al2Cl6 in the vapour. Each Al atom acts as an acid towards a Cl atom initially belonging to the other Al atom. Aluminum chloride is widely used as a Lewis acid catalyst for organic reactions.
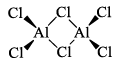
Alkyl aluminum dimers are similar in structure to the analogous dimeric halides but the bonding is different. In the halides, the bridging Al—Cl bond involves an electron pair. In the alkyl aluminum dimers, the Al—C—Al bonds are longer than the terminal Al—Cl bonds, which suggests that they are 3c—2e bonds, with one bonding pair shared across the Al—C—Al unit, somewhat analogous to the bonding in diborane, B2H6.
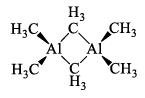
(iv) Alumns
M2SO4, M’2(SO4)2.24H2O
Where M = Na+, K+, Rb+, Cs+, As+, Tl+, NH4+
M’ = Al+3, Cr+3, Fe+3, Mn+3, Co+3
K2SO4.Al2(SO4)3.24H2O Potash alum
(NH4)2SO4.Al2(SO4)3.24H2O Ammoniumalum
K2SO4.Cr2(SO4)2.24H2O Chromealum
(NH4)2SO4.Fe2(SO4)2.24H2O Ferricalum
Preparation: Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
Al2(SO4)3 + K2KO4 + aq. Solution → crystallize
Properties
It is a white amorphous powder insoluble in water but soluble in acids (forming eg., AlCl3) as well as alkalies (forming NaAlO2). Thus amphoteric in nature. It is a polar covalent compound.
|
48 videos|92 docs|41 tests
|
FAQs on Group 13 Elements: Boron Family - Inorganic Chemistry
| 1. What is the inert pair effect in p-block elements? |  |
| 2. What are the atomic and physical properties of group 13 elements? |  |
| 3. What are the properties of boron? |  |
| 4. What are some commonly known compounds of boron? |  |
| 5. How is aluminum related to the boron family (group 13 elements)? |  |

|
Explore Courses for Chemistry exam
|

|


















