Group 15 Elements: Nitrogen Family | Inorganic Chemistry PDF Download
GROUP 15TH ELEMENTS (NITROGEN FAMILY)
Element | N | P | As | Sb | Bi | |
Atomic number | 7 | 15 | 33 | 51 | 83 | |
Atomic mass | 14.91 | 30.97 | 74.92 | 121.76 | 208.92 | |
Electronic configuration | [He] 2s2 2p3 | [Ne] 3s2 3p3 | [Ar] 3d10 4s2 4p3 | [Kr] 4d10 5s2 5p3 | [Xe] 4f14 5d10, 6 s2 6p3 | |
Covalent Radius/pm | 70 | 110 | 120 | 140 | 150 | |
Lonization enthalpy/(KI mol–1) | 1 | 1402 | 1012 | 947 | 834 | 703 |
Electronegativity | 3 | 2.1 | 2 | 1.9 | 1.9 |
NITROGEN
Preparation
1. NH4NO2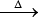 N2↑+ 4H2O + Cr2O3
N2↑+ 4H2O + Cr2O3
2. (NH4)2Cr2O7 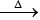 N2↑ + 4H2O + Cr2O3
N2↑ + 4H2O + Cr2O3
3. 8NH3 + 3Cl2 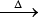 6NH4Cl + N2
6NH4Cl + N2
Properties
(1) N2 is a colourless odourless gas insoluble in water.
(2) It is absorbed by heating with Mg and Al. The nitrides formed thus react with water to form NH3.
3Mg + N2→Mg3N2 (+6H2O)→3Mg(OH)3 + 2NH3↑
2Al + N2→2AlN (+6H2O)→2Al(OH)3 + 2NH3↑
(3) Reaction with H2 at 200 atm and 500 °C and in the presence of iron catalyst and molybdenum promotes N2 combination with H2 reversibly to form ammonia. The process in called Haber’s Process and is the industrial method of manufacturing ammonia. The reaction is exothermic.

(4) Reaction with oxygen: When air free from CO2 and moisture is passed over an electric are at 2000-3000°C nitric oxide is formed. This reaction its endothermic.
N2 + O2 → 2NO
AMMONIA (NH3)
Preparation:
1. NH4Cl + NaOH 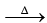 NH3 ↑ + NaCl + H2O
NH3 ↑ + NaCl + H2O
2. (NH4)2SO4 + 2NaOH 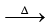 2NH3 ↑ + Na2SO4 + 2H2O
2NH3 ↑ + Na2SO4 + 2H2O
3. NH4NO3 + NaOH 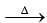 NH3 ↑ + NaNO3 + H2O
NH3 ↑ + NaNO3 + H2O
Haber’s process: N2 + 3H2 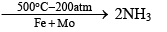
Cyanamide Process
(i) CaO + 2C + N2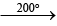 CaCN2 + CO ↑
CaCN2 + CO ↑
(ii) CaCN2 + 3H2O → CaCO3 + 2NH3 ↑
Properties
1. Na + NH3 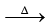 NaNH2 + 1/2H2
NaNH2 + 1/2H2
2. 4NH3 + 5O2 4NO + 6H2O
4NO + 6H2O
3. CuSO4 + 2NH4OH  Cu(OH)2 ↓ (blue) + (NH4)2SO4
Cu(OH)2 ↓ (blue) + (NH4)2SO4
4. Cu(OH)2 + (NH4)2SO4 + 3NH4OH (excess)  [Cu(NH3)4]SO4 (deep blue solution) + 4H2O
[Cu(NH3)4]SO4 (deep blue solution) + 4H2O
OXIDES OF NITROGEN
Oxides of nitrogen | Structure | Physical state | Colour of gas |
N2O | 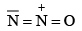 | Gas | Colurless |
NO | 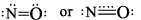 | Gas | Colourless |
N2O3 | 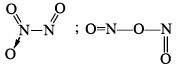 | Blue liquid (–30°C) | |
NO2 | 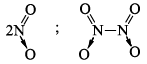 | Gas | Brown |
N2O5 | 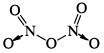 | Colourless solid | –(no existence in gas phase) |
Preparation:
1. NH4NO3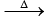 N2O + 2H2O
N2O + 2H2O
2. 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O
Properties
1. Oxides of nitrogen are all oxidizing a gents.
2. (a) N2O is isoelectronic with CO2 and also has linear structure. However, unlike CO 2, N2O has a small dipole moment.
(b) No has total of 15 electrons. This is an odd electron molecule. In the gaseous state, it is paramagnetic. However the liquid and the solid states are diamagnetic because lose dimmers are formed canceling out the magnetic effects of unpaired electrons.
The bonding in NO is best described by the molecular orbital theory. Its electronic configuration is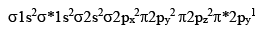
This gives a bound order of 2.5. If the u npaired electron occu pying he a nti bonding π*2py orbital is removed, nitrosonium ion NO+ is formed and bond order becomes 3. This is reflected in the thortning of the bond length from 115 pm in NO to 106 pm in NO+. Nitrosonium ion is stable and forms salts like NO+Cl-. It is isoelectronic with CO and forms complexes with transition elements. The brown ring formed in the test for nitrates is due to the formation of a complex of iron [Fe(H2O)5NO]2+
(c) NO2 with 23 electrons is aga in an odd electron molecule. In the gaseou s state it is para magnetic. On cooling, the gas condenses to a brown liquid and eventually to a colourless solid both of which are diamagnetic due to dimerisation. NO2 molecule is angular with ONO angle of 134°. The O —N bond length is 120 pm. Intermediate between a single and double bond. The odd electron is o nitrogen. The dimer has been shown to have a linear structure. The N—N bond length is very large 174 cm making this a very weak bond liquid N2O4 undergoes self-ionizatin to form NO+ and NO3– ions and (therefore it has been extensively studied as a non-aqueous solvent
(d) Solid N2O5 exists in the ionic form NO2+ NO3– in the gaseous form, the discreate N2O5 molecules have a N—O—N bond angle close to 112°
Properties:
N2O: (a) Reduction: Cu(hot) + N2O → CuO + N2
(b) Oxidation: 2KMnO4 + 2H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 10NO
NO: (a) Oxidizing properties (Reduction of NO)
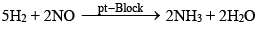
(b) Reducing properties (oxidation of NO):
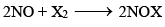
6KMnO4 + 9H2SO4 + 10 NO → 3K2SO4 + 6MnSO4 + 4H2O + 10HNO3
NO2: It behaves both like HNO2 as a reducing agent and like HNO3 as an oxidizing agent according to following reactions respectively.
2KMnO4 + 3H2SO4 + 10NO2 + 2H2O → K2SO4 + 2MnSO4 + 10HNO3
SO2 + H2O + NO2 → H2SO4 + NO
OXYACIDS OF NITROGEN
NITROUS ACID (HNO2)
Preparation
1. Ba(NO3) + H2SO4 → 2HNO2 + BaSO4↓
Properties
(i) Nitrous acid and nitrites are good oxidizing agents and covert iodides to iodine, ferrous salts to ferric stannous to stannic and sulphites to sulphates eg.
2KI + 2HNO2 + 2HCl → 2H2O + 2NO + 2KCl + I2
(ii) With strong oxidizing a gents like KMnO4, nitrou s a cid a nd nitrites function a s redu cing agents a nd get oxidized to NO3– ions
2KMnO4 + 5KNO2 + 6HCl → 2MnCl2 + 5KNO3 + 3H2O + 2KCl
NITRIC ACID (HNO3)
Preparation
HNO3 is exclu sively manu factured by the Ostwald process. In this process NH3 is catalystically oxidized to NO over a Pt—Rh catalyst at 1200 K
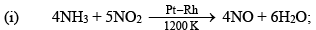 ΔH = -904 Kj
ΔH = -904 Kj
The mixture is then diluted with air NO combine s with O2 to give NO2 which is absorbed in water to give HNO3 and NO which is then recycled.
(ii) 2NO + O2 → 2NO2
(iii) 3NO2 + H2O → 2HNO3 + NO
Properties
(i) Thermal stability
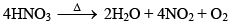
(ii) Oxidizing Properties:
(a) H2S + 2HNO3 (conc.)→2H2O + 2NO2 + S ↓
(b) 3H2O + 2HNO3 (dilute) → 4H2O + 2NO + 3S
(c) SO2 + 2HNO3 (conc.) → H2SO4 + 2NO2
(d) 3SO2 + 2H2O + 2HNO3 (dilute)→ 3H2SO4 + 2NO
(e) Zn + 4HNO3(conc.) →Zn(NO3)2 + 2H2O + 2NO2
(f) 4Zn + 10HNO3(dil.) → 4Zn(NO3)2 + 5H2O + N2O
(g) Cu + 4HNO3(conc.) → Cu(NO3)2 + 2NO2 + 2H2O
(h) 3Cu + 8HNO3 (v. dil) → 3Cu(NO3)2 + NO + 4H2O
PHOSPHORUOUS (P)
It is a very reactive non-metal. It catches fire in air. It occurs in nature in the form of stable phosphates. The important minerals are:
(i) Phosphorite, Ca3(PO4)2
(ii) Vivianite, Fe3(PO4)28H2O
EXTRACTION
Ca3(PO4)2 + 2SiO2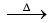 3CaSiO3 + P2O5
3CaSiO3 + P2O5
P2O5 + 5C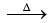 2P↑ + 5CO↑
2P↑ + 5CO↑
ALLOTROPIC FORMS OF PHOSPHOROUS
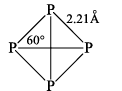
(i) WHITE OR YELLOW PHOSPHORUS (P4)
Properties
(1) It is white-too-transparent and soft waxy solid
(2) It is soluble in CS2 but insoluble in water.
(3) It glows in dark due to slow oxidation producing yellowish-green light. This phenomenon is called phosphorescence.
(4) White phosphrous is poisonous.
(5) It runs yellow after some time, it is called yellow phosphorus.
(6) It undergoes oxidation in the presence of air which slowly rises its temperature and due to its low ignition temperature (30°C) after a few moments it catches fire spontaneously. Due to this reason it is stored under water.
(ii) RED PHOSPHORUS
Preparation
When white phosphorus is heated in the atomosphere of CO2 or coal gas at 250°C red phosphorus is produced. This red phosphorus may still contain some white phosphorus which is removed by boiling the mixture with NaOH where white phosphorus is converted into PH3 gas but red phosphorus remains inert.
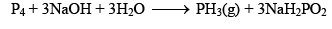
It is also prepared by heating white phosphorus with a few crystals of iodine catalyst at 250°C under high pressure.
Properties
It is red crystalline solid having a density of 2.2 g/cc. It is less reactive then white phosphorus and does not dissolve in liquid CS2. It does not catch fire at room temperature because its ignition temperature is 260°C. It is a polymeric substance forming linear chains like this.
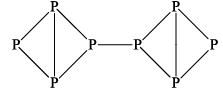
(iii) BLACK PHOSPHOROUS
Preparation
When white phosphorus is heated at about 200°C under very high pressure then black phosphorous is produced.
Properties
It is an electrical conductor resembling graphite in this respect and also in its flakiness and luster. It is often called metallic phosphorous. Its density (2.69 g/cc) is higher than of red phosphorous. It is insoluble in CS2. It has layered structure like graphite.
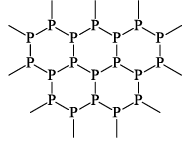
(iv) BROWN PHOSPHOROUS
Above 1600°C P4 molecules break into P2 molecules. Rapid cooling of this vapour gives brown phosphorus which probably contains P2 molecules.
(v) SCARLET PBHOSPHROUS
This allotrope is obtained in the form of an amorphous scarlet powder by boiling a 10 percent solution of phosphorus in phosphorus tribromide for about 10 hours. Pure scarlet phosphorus may be prepared by heating phosphorus tribromide with mercury at 240°C.
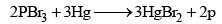
Scarlet phosphorus
Scarlet phosphorus resembles the red variety in its physical properties and the white phosphorus in its chemical properties. It is, however, only slowly oxidized in air.
(iv) VIOLET PHOSPHORUS
This variety is obtained by heating white phosphorus with a trace of sodium at 230°C under high pressure. It is crystalline in structure.
CHEMICAL PROEPERTIES OF PHOSPHORUS
Reactivity of the various allotropic forms of phosphorus towards other substances decreases in the order.
Brown > white > red > black, the last one being almost inert & thermodynamically most stable form of phosphorus.
(i) Action of air: White phosphorus burns in air to form phosphorus trioxide and pentoxide.

Red and other forms of phosphorus also burn in air or oxygen but on heating.
(ii) Halide: Nitrogen is unable to form pentahalides because the second shell contains a maximum of eight electrons, i.e., four bonds. The subsequent elements have suitable d orbitals and form the following pentahalides PX5.
PCl5 + 4H2O→H3PO4 + 5HCl
Despite the existence of pentahalides no hydrides MH5 are k nown. To attain the five valent state, d orbitals must be used. Hydrogen is not sufficiently electronegative to make the d orbitals contract sufficiently, through PHF4 and PH2F3 have been isolated.
(iii) Action with Non metals: When heated with non-metals phosphorous forms compounds PX3, PX5, P2S3 and P2S5
2P + 3X2→2PX3,
2P + 5X2→2PX5. (where X = Cl, Br and I)
(iv) Action with metals: Alkali metals when heated with white phosphorus in vacuum produce alkali metal phosphide, which react with water to form phosphine gas.
3M + P 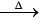 M3P
M3P
M3P + 3H2O 3MOH + PH3 ↑ (where M = Na, K etc.)
3MOH + PH3 ↑ (where M = Na, K etc.)
(v) Action of NaOH: When white phosphorus is heated with NaOH solution, phosphine gas is evolved.
P4 + 5HNO3 →3NaH2PO2 + PH3 ↑
(vi) Action of conc. HNO3/H2SO4: When heated with conc. HNO3 phosphorous is oxidized to H3PO4.
P + 5HNO3→H3PO4 + 5NO2 ↑ + H2O
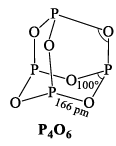
PHOSPHORUS TRIOXIDE (P2O3)
It is prepared by burning phosphorus in a limited supply of oxygen when gaseous P4O10 and P4O6 are formed.
Properties
(i) It is colourless crystalline solid having mp 23.8 °C and bp 178 °C.
(ii) It dissolves in cold water to form phosphorous acid, it is thus the anhydride of phosphorous acid.
P2O3 + 3H2O → 2H3PO3
(iii) It burns in Cl2 gas forming phosphorous oxytricholoride (POCl 3) and phosphoryl chloride (PO2Cl)
P2O3 + 2Cl2 →POCl3 + PO2Cl
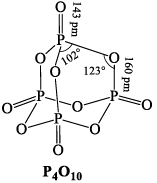
PHOSPHOROUS PENTOXIDE (P2O5)
Preparation
It is obtained by burning phosphorous in air.
P4 + 5O2 →P4O10
Properties
(i) It is a white powder acidic in nature and is the anhydride of orthophosphoric acid. Its empirical formula is P2O5 and its molecular formula is P4O10.
(ii) Action of water: It dehydrates in water with hissing sound forming metaphospharic acid and finally orthophosphoric acid
P4O10 + 2H2O→4HPO3;
HPO3 + H2O →H3PO4
(iii) Dehydrating power: It dehydrates conc. H2SO4 and cocnc. HNO3 to SO3 and N2O5 respectively.
OXY-ACID OF PHOSPHOROUS
PHOSPHOROUS ACID (H3PO3)
Preparation
(i) By dissolving P2O3 in water
P2O3 + 3H2O→2H3PO3
(ii) By hydrolysis of PCl3 with water.
PCl3 + 3H2O → H3PO3 + 3HCl
Properties
(i) It is a white crystalline solid soluble in water and having melting point of 74°C
(ii) It is a weak acid and reducing a gent.
(iii) 4H3PO3 3H3PO4 + PH3 (Disproportionation)
3H3PO4 + PH3 (Disproportionation)
(iv) H3PO3 + 3PCl3 → PCl3 + 3POCl3 + 3HCl
(v) It is a strong reducing agent
2AgNO3 + H3PO3 + H2O → 2Ag + 2HNO3 + H3PO4
ORTHOPHORSPHORIC ACID (H3PO4)
Preparation
(i) Ca3(PO4)2 + 3H2SO4→2H3PO4 + 3CaSO4
(ii) P4O10 + 6H2O→4H3PO4
(iii) P4 + 20HNO3 →4H3PO4 + 20NO2 + 4H2O
Properties
Reacting of H3PO4: The simplest phosphorous acid is H3PO4 (orthophosphoric acid). The acid contains three replaceable H atoms and is tribasic.
(i) Pure orthophosphoric acid is a white crystalline solid highly soluble in water having melting point of 42°C. It is a weak acid. It forms two acid salts and one normal salt. NaH2PO4 is sodiu m dihydrogen phosphate, Na2HPO4 is disodium hydrogen phosphate & Na3PO4 is sodium orthophosphate.
(ii) Action of Heat:
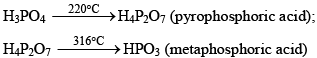
(iii) Neutralization with alkalis or bases:
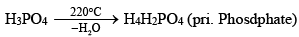
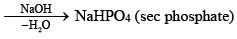
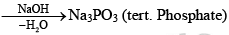
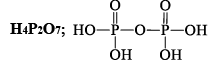
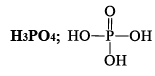
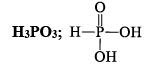
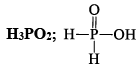
Polyphosphonitrilic chlorides (PNCl2) (n = 3 to 7)
These polymers are also called inorganic rubbers. These compounds are can be represented by the General structure,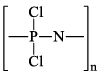
Preparation:
(i) (NPCl)3 and (NPCl2)4 can be prepared by the ammonolysis of PCl5.
3PCl5 + 3NH3→(NPCl2)3 + 9HCl
4PCl5 + 4NH3 → (NPCl2)4 + 12HCl
(ii) These compou nds can be prepared by the reaction between PCl5 and NH4Cl in presence of C2H4Cl2 or C6H5Cl or by heating PCl5 with solid NH4CH at 145-160°C
nPCl5 + nNH4Cl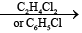 (NPCl2)n + 4HCl
(NPCl2)n + 4HCl
nPCl5 + nNH4Cl(s)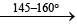 (NPCl2)n + 4nHCl
(NPCl2)n + 4nHCl
Properties:
Reactions involving replacement of Cl-atom of P—Cl bond:
(i) (NPCl2)3 + 6NaF  (NPF2)3 + 6NaCl
(NPF2)3 + 6NaCl
(ii) [NPCl2)3 + 2NaOCH3 [ NP(OCH3 )2 ] 2NaCl Tri(dimethoxy) phosphazine
[ NP(OCH3 )2 ] 2NaCl Tri(dimethoxy) phosphazine
(iii) [NPCl2)3 + 6C6H5MgBr 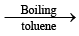 [NP(C6H5)2]3 + 3MgCl2 + 3MgBr2
[NP(C6H5)2]3 + 3MgCl2 + 3MgBr2
(iv)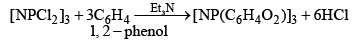
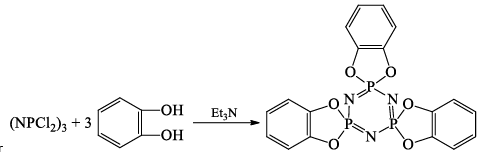
Or
Hydrolysis:
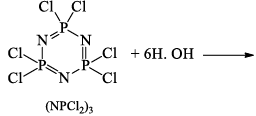
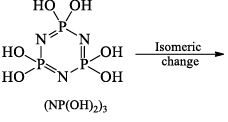
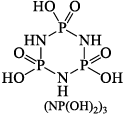
Reaction with benzene, C6H6 (Friedel-Crafts reaction):
N3P3Cl6 + 2C6H6  N3P3Cl4 (C6H5)2 + 2HCl
N3P3Cl4 (C6H5)2 + 2HCl
PbF2 fluorinates N3P3Cl6 (trimer) giving ultimately N3P3F6.
N3P3Cl6 + 3PbF2 →N3P3F6 + 3PbCl2
Structure of (NPCl2)3 molecule: X-ray analysis has shown that (NPCl2) molecule has a planar sixmembered ring structure (Structural) in which each N -atom is sp2 hybridized and each P-atom is sp3 hybridized. The lone pair of electrons on each N -atom resides in one of the three sp2 hybrid orbitals. It is this lone pair of electrons which makes (NPCl2)3 molecule to show basic properties. The bond angles are as shown in the structure. Resonance structure can also be drawn as in case of C6H6 molecule, indicating aromaticity in the ring.
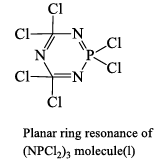

Unlike benzene which involves extensive (pπ- pπ) bonding. (N3P3Cl6) molecule involves (dπ-pπ) bonding. The extent (dπ-pπ) bonding appears to be quite appreciable as the N—P distance (1.6 Å) is considerably shorter than the N—P single bond distance (1.75-7.80 Å). Whether there is complete delocalization of π-electron charge cloud on all the ring a to ms as in C6H6 molecule or there are intensely-localized islands of the electron cloud within the PNP segments cannot be answered with certainity.
Structure of (NPCl2)4 molecule: This molecule has a tube-like puckered structure.
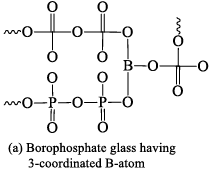
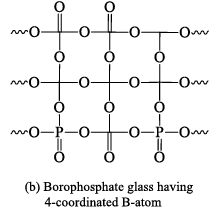
Borophosphate glasses
These are formed by heating H3PO4, B2O3 and alkali metal carbonates or oxides at 700 °C. Borophosphate glasses are of the following three types. (i) Those which contain excess of alkali over P2O5 in this variety all the B atoms are present a s trigonal BO3 groups.
(ii) Those which contain excess of P2O5 over alkali. These borophosphate glasses are called acidic borophosphate glasses. If there is less than 10 mole percent of B2O3 all B-atoms are four coordinated.
(iii) Those which contain P2O5 and alkali in equivalent proportions. The number of coordinated B -atoms decreases steadily with the increase in the content of B2O3 and becomes almost zero at about 47 mole per cent of B2O3.
Structure of borophosphate glass having three and four coordinate B atoms are shown below in following figure.
|
48 videos|92 docs|41 tests
|
FAQs on Group 15 Elements: Nitrogen Family - Inorganic Chemistry
| 1. What are the elements in Group 15 of the periodic table? |  |
| 2. What are the common properties of Group 15 elements? |  |
| 3. What is the significance of nitrogen in biological systems? |  |
| 4. How do Group 15 elements react with oxygen? |  |
| 5. What are some uses of Group 15 elements? |  |

|
Explore Courses for Chemistry exam
|

|













