Halogenation Reaction - Class 11 PDF Download
Ref: https://edurev.in/question/527313/Three-steps-in-halogenation-reaction-area-Initiation-propagation-and-combustionb-Initiation-propagat
when we started to go through free radical substitution reactions, you might recall that we looked a reaction like this one:
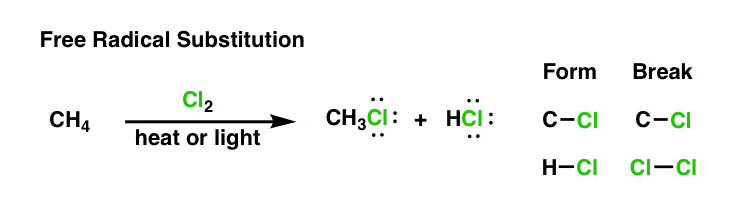
Now that we know a little more about what free radicals are and their key properties, the question for today is, “how does this reaction work?“. We’re going to go through the key steps of this reaction and learn that they are composed of three key phases: initiation, termination, and propagation.
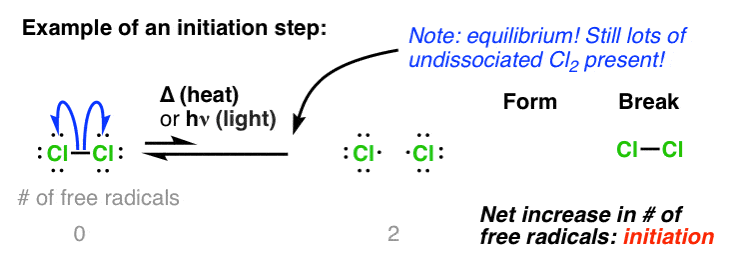
In the last post, we talked about how nothing happens unless heat or light is applied. That’s because either of these energy sources can lead to homolytic cleavage of relatively weak bonds like Cl-Cl to give free radicals [i.e. Cl• ]
Every free radical reaction begins with a step where free radicals are created, and for that reason this initial step is called initiation.
Here’s the equation. Two things to note:
- The reaction is an equilibrium – at any given time there is only a small concentration of the free radical present (but this will be enough, as we shall see)
- Note that there is a net increase of free radicals in this reaction. We’re going from zero(in the reactants) to two (in the products).
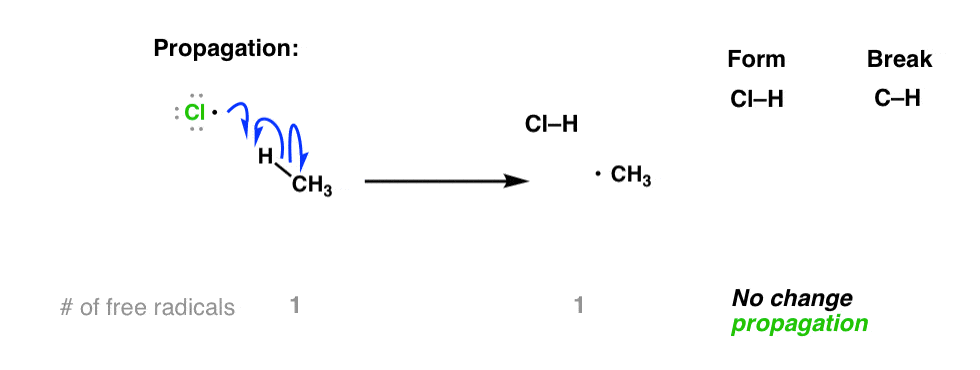
 It’s only once the free radicals are present that our substrate (CH4 in our example) gets involved. Chlorine radicals are highly reactive, and can combine with a hydrogen from methane to give the methyl radical, •CH3
It’s only once the free radicals are present that our substrate (CH4 in our example) gets involved. Chlorine radicals are highly reactive, and can combine with a hydrogen from methane to give the methyl radical, •CH3
If you count the number of free radicals in this equation, you’ll note that there’s one in the reactants and one in the products. So there is no net increase in the number of free radicals.
This type of step is referred to as “propagation”.
If you’re keeping score, by this point you should be able to see that there’s only one bond left to form before our reaction is complete. We need to form a C–Cl bond.
It’s here where it’s easy to make a little mistake. Seeing that there’s two chlorine radicals formed in the initiation step, it would seem natural to bring together the methyl radical and the chlorine radical to form CH3–Cl . Right?????
Nooooo!
Note the number of free radicals has decreased here, not stayed the same. It can’t be propagation!

In fact, we can do the proper “propagation” step this way: Take the methyl radical, and it reacts with the Cl2 still present. This gives us CH3Cl and the chlorine radical. Note that there has been no net change in the number of free radicals, so this is still a “propagation”.
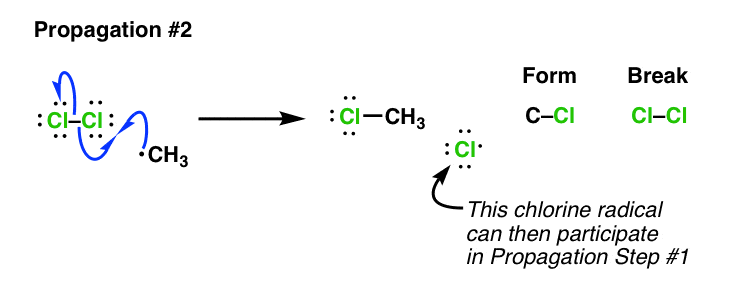
Note again that we are forming a chlorine radical! What’s so crucial about this? It’s crucial because this chlorine radical can then perform Propagation Step #1 on a new molecule of our substrate (CH4), continuing the process. It’s a chain reaction – once generated, chlorine radical is catalytic. That’s why we only need a small amount of chlorine radical for this reaction to proceed.
Can this chain reaction go on forever? No.
Let’s think about two limiting cases. If the concentration of Cl2 is low relative to CH4 (in other words, Cl2 is our limiting reagent) then the rate of Propagation Step #2 will slow down as its concentration decreases. Without any Cl2 to react with, our •CH3 radicals can just combine with another free radical (such as •Cl) to give CH3Cl, for example. There is essentially no barrier to this reaction. Note that here the number of free radicals decreases from 2 to zero. This is called termination.

It’s also possible for two methyl groups to combine together to give CH3–CH3 ; this is also termination!
Let’s put all of these steps together so we can clearly see the initiation, propagation, and termination steps.
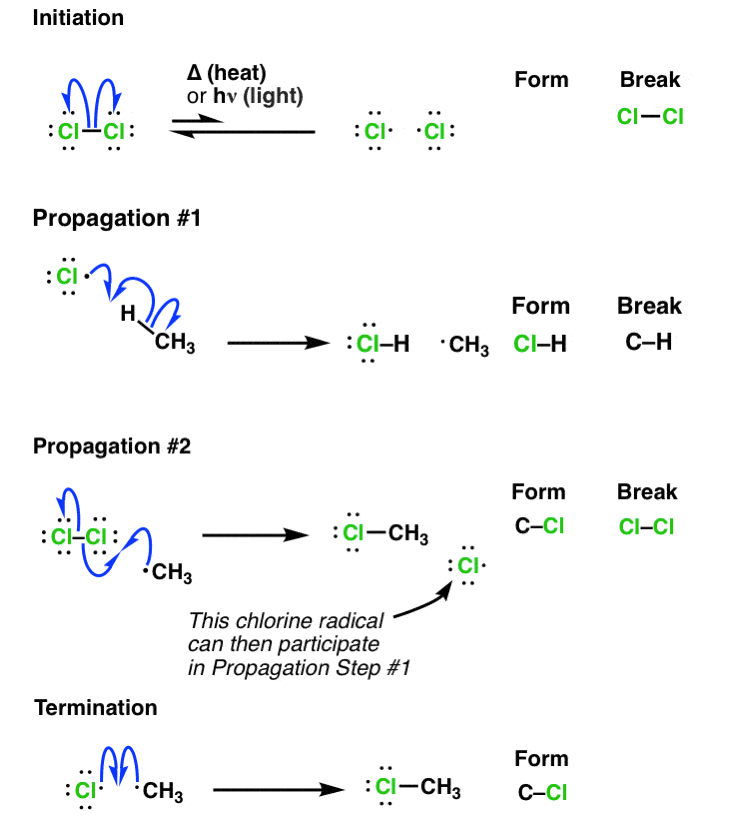
These three types of steps are encountered in every free-radical reaction.
The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination.
- Intiation -> net formation of radicals
- Propagation -> no change in the number of free radicals
- Termination -> net destruction of free radicals
We’ll leave with two teasers for future posts.
First… note that here we’re using CH4, where every C–H bond is identical. What might happen if we used an alkane where all the C–H bonds aren’t equal… like propane, or pentane, for example?
Secondly, this reaction fails spectacularly when Br2 is used instead of Cl2 for the reaction of CH4. However, we’ll see that Br2 can work in certain special cases.


















