Heat and Thermodynamics | General Awareness for SSC CGL PDF Download
Introduction
Heat and temperature are fundamental concepts in physics that describe the energy related to the warmth of objects and their changes. Heat involves energy transfer due to temperature differences, while temperature measures how hot or cold something is. Understanding these concepts is crucial for various applications, from everyday life to scientific studies.
Heat
Heat is a type of energy that creates the feeling of warmth. Its standard unit of measurement is the joule, though the calorie is also commonly used (with 1 calorie equaling 4.2 joules). When a body is heated, it can undergo various changes, including expansion, contraction, changes in state, and alterations in electrical properties. Heat always flows from a hotter object to a cooler one. Additionally, the heat energy transferred between bodies can be converted into other forms of energy, such as mechanical or electrical energy.
Temperature
- Temperature is measure of hotness or coldness of a body.
- The heat flows from one body to another due to the difference in their body temperature.
Scale of Temperature
To measure the temperature of a body following temperature scales are used.
- Celsius scale of temperature ice point is 0°C Boiling point of water = 100°C
- Fahrenheit scale of temperature ice point = 32° F Boiling point of water = 212° F
- Kelvin or absolute scale of temperature ice point = 273 ° K Boiling point of water = 373 ° K
- Reaumur scale of temperature ice point is 0° R, Boiling point of water = 80° R
- Rankine scale of temperature ice point = 491.67 °R Boiling point of water = 671.641° R
Where, Lower Fixed Point (LFP) = lce point and Upper Fixed Point (UFP) = Boiling point of water.
Relation between Different Scales of Temperature
Different scales of temperature are related as follows:
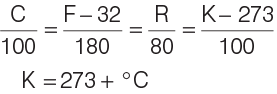
- At temperature − 40° C = − 40° F
- The temperature at which the three phases of water remains at equilibrium is called triple point of water (273.16 K)
Thermometers
Instruments used to measure the temperature of a body are called thermometers. There are three main types of thermometers:
- Clinical Thermometer: This type measures human body temperature, with a range from 96°F to 110°F (35°C to 43°C).
- Electronic Thermometer: This thermometer uses thermistors or thermoresistors as its primary components and can measure temperatures from –40°F to 450°F.
- Other Thermometers: These include devices like constant volume gas thermometers and platinum resistance thermometers.
Clinical thermometers typically measure temperature in degrees Fahrenheit (°F). Mercury is commonly used in thermometers, covering a temperature range from –30°C to 300°C. The thermometer was first developed by Galileo, who discovered that gases expand when heated.
Thermal Expansion
The expansion of a body caused by heat is known as thermal expansion.
Thermal Expansion of Solids
Thermal expansion of solids is of three types
1. Expansion in length on heating, is called linear expansion. The increase in length of a rod of unit length of a substance due to increase in its temperature by 1° C is called the coefficient of linear expansion of the substance of that rod. It is represented by α. – Its unit is ° C−1.
– Its unit is ° C−1.
2. Expansion in area on heating, is called superficial expansion. Coefficient of superficial expansion is given as – Its unit is ° C−1.
– Its unit is ° C−1.
3. Expansion in volume on heating, is called volume expansion or cubical expansion. Coefficient of volume or cubical expansion is given as
– Its unit is ° C−1
Relation between Coefficients of Expansions
- Coefficients of thermal expansions are related as
β = 2α and γ = 3 α
and α : β : γ = 1 : 2 : 3 - In laying a railway line, a small gap is left in between two iron rails otherwise railway line will become curved on heating in summer.
- Telephone wires are not tighten on poles because in winter, wires get contract and can break.
Thermal Expansion of Liquids
- In liquids, only expansion in volume takes place on heating.
Expansion of liquid is of two types — - When expansion of the container, containing liquid, on heating, is not taken into account, then observed expansion is called apparent expansion of liquids.
- Coefficient of apparent expansion of liquid.

- When expansion of the container, containing liquid, on heating, is also taken into account, then observed expansion is called real expansion of liquids.
- Coefficient of real expansion of a liquid

γr γa + γg
where, γr and γa are coefficients of real and apparent expansion of liquids and γg = coefficient of cubical expansion of the container.
Anomalous Expansion of Water
When temperature of water is increased from 0° C, then its volume decreases up to 4° C, becomes minimum at 4° C and then increases. This behavior of water expansion around 4° C is called, anomalous expansion of water.
Thermal Expansion of Gases
here are two types of coefficient of expansion in gases
- At constant pressure, the change in volume per unit volume per degree celsius, is called volume coefficient (γV).
- At constant volume, the change in pressure per unit, pressure per degree celsius, is called pressure coefficient (γp).
Calorimetry
- Amount of heat required to raise the temperature of 1 g of water by 1°C is called 1 calorie.
- Calorimetry states that heat lost by hotter body equals the heat gained by colder body.
- A both or container in which calorimetry (process) takes places is called calorimeter.
Specific Heat
- The amount of heat required to raise the temperature of unit mass (m) of a substance through 1° C, is called its specific heat (s). — It is denoted by s and its unit is ‘cal/g°C or Joule/g°/ C.
- The specific heat of water is 4200 J / kg1 / ° C or 1000 cal/ g1/ ° C− , which is high compared with most other substances. Therefore, water is used as coolant in radiator in vehicle and hot water is used for the fermentation.
- Heat energy given or taken to change the temperature of a body is given by
Q = ms∆θ
where, m = mass of the body
and ∆θ = change in temperature. - At constant volume, the amount of heat required to raise the temperature of 1 g of a gas by 1° C is called specific heat at constant volume (CV). Its unit is cal/g/°C.
- At constant pressure, the amount of heat required to raise the temperature of 1g of a gas by 1°C is called specific heat at constant pressure (Cp). Its unit is cal/g/°C.
- The amount of heat required to raise the temperature of 1 mole of a gas by 1°C is called molar specific heat.
Latent Heat
- The heat energy absorbed or released at constant temperature per unit mass for change of state, is called latent heat.
- It is denoted by L and its SI unit is cal/g or kcal/kg.
- Heat energy absorbed or released during change of state is given by
Q = mL
where, m = mass of the substance. - Latent heat of fusion of ice is 80 cal/g.
- Latent heat of vaporisation of steam is 536 cal/g
Thermodynamics
Thermodynamics is the branch of physics that explores the relationship between heat energy and various forms of energy.
- Zeroth Law: The Zeroth Law of Thermodynamics addresses the concept of thermal equilibrium.
- First Law: According to the First Law of Thermodynamics, the heat added to a substance is equal to the sum of the change in its internal energy and the work done by the substance.
- Second Law: The Second Law states that work can be converted into heat and vice versa, but this conversion is never 100% efficient. Kelvin’s statement asserts that it is impossible for a machine operating in a cyclic process to convert all heat into work. Clausius’s statement explains that heat cannot transfer from a colder body to a hotter body on its own, a principle on which refrigerators are based.
- Heat Engine: A heat engine is a device that converts heat into mechanical work. There are two main types of heat engines: internal combustion and external combustion engines.
- Car Engine: Car engines use a coolant mixed with water, such as ethylene glycol or potassium dichromate, to mitigate issues like corrosion and rusting.
- Carnot’s Theorem: Carnot’s Theorem describes the maximum possible efficiency of a heat engine, based on the Carnot cycle.
- Entropy: Entropy measures the molecular disorder within a system and is a thermodynamic function dependent solely on the system's temperature.
- Evaporation: Evaporation is the process by which molecules gradually escape from the surface of a liquid.
- Rate of Evaporation: The rate of evaporation for a given liquid depends on factors such as temperature and the area of the evaporating surface.
- Refrigerator: A refrigerator cools items through the evaporation and compression of a volatile liquid within a copper coil.
Humidity
- Humidity refers to the presence of moisture in the atmosphere.
- Absolute humidity is the measure of the amount of water vapor present in a unit volume of the atmosphere.
- Relative humidity is the ratio of the mass of water vapor present in a specific volume of air to the mass of water vapor needed to saturate the same volume of air at the same temperature, expressed as a percentage.
- Relative humidity is measured using a hygrometer.
- A relative humidity of around 50% is considered comfortable at temperatures between 22°C and 25°C.
- When relative humidity is very low, lips can become dry and may crack.
- High relative humidity prevents sweat from evaporating easily, leading to discomfort.
- Air conditioning creates a comfortable environment by regulating both temperature and humidity.
Transmission of Heat
Heat can be transferred from one place to another by process of transmission. There are three methods of transmission of heat.
Conduction
- The mode of transmission of heat in solids from higher temperature part to lower temperature part without actual movement of the particles, is called conduction.
- Transmission of heat in solids takes place mainly through conduction.
- Metals are good conductors of heat.
- Wood, cotton, wool, glass are bad conductors of heat, dry air is also a bad conductor of heat.
- Woollen clothes do not allow the heat of our body to escape and therefore we feel warm.
- On a cold night two thin blankets give more warmth than a single thick blanket because the layer of air between the two blankets works as a better insulator.
- Refrigerators and ice-boxes have double walls having thermocol between them which minimise heat gain by conduction.
Convection
- The mode of transmission of heat in fluids (liquids and gases) due to actual movement of the particles, is called convection.
- In liquids and gases, heat is transmitted by convection.
- When a liquid in a vessel is heated at the bottom, the liquid at bottom gets heated and expands.
- Due to its lower density, hot liquid rises and its place is taken by cold liquid from above. Convection currents are set up in the liquid until the temperature of the whole liquid becomes same.
- The cooling unit in a refrigerator is fitted near the top as cold air move downward and keeps cool the whole interior.
- Radiator in a motor car works on the principle of convection.
- Sea Breeze During day time, the seashore warms up much faster than sea water. Hot air over the seashore rises and cooler air from sea water moves towards seashore to take its place resulting in a sea breeze.
- Land Breeze At night, land cools faster than sea water . Now hot air over sea water rises and cooler air from land moves towards sea to take its place and resulting in a land breeze.
- Cloudy night are warmer than clear night because clouds reflect the radiations emitted by the earth at night and keep it warm.
Radiation
- The process of heat transmission in the form of electromagnetic waves, is called radiation.
- Radiation does not require any medium for propagation and it propagates without heating the intervening medium.
Black Body
A black body is defined as an idealized object that absorbs all incident radiation perfectly. The ratio of heat absorbed by the body to the total incident radiation is known as its absorptive power (α), which has no units. The heat radiation emitted per unit area of the body’s surface at a specific temperature is referred to as the emissive power. The unit for emissive power is J/m2·s. Kirchhoff’s law states that the ratio of emissive power to absorptive power for any body is consistent and equals the emissive power of a black body.
In terms of color and heat absorption, white colors are poor absorbers but good reflectors of heat, while black colors are excellent absorbers and poor reflectors. Hence, light-colored clothing is more comfortable in hot weather, whereas dark-colored clothing is preferable in cold weather.
Stefan’s Law
Stefan’s Law asserts that the energy (E) radiated per second per unit area by a perfect black body is directly proportional to the fourth power of its absolute temperature (T). This relationship is expressed as E ∝ T4. In essence, good absorbers of heat are also efficient emitters, while poor absorbers are poor emitters.
Wien’s Displacement Law
Wien’s Displacement Law indicates that the wavelength (λm) at which the emission of a black body radiation is at its peak is inversely related to the body’s absolute temperature (T). As the temperature of a black body increases, it first emits light that appears red, then orange, followed by yellow, and eventually shifts to blue and violet.
Greenhouse Effect
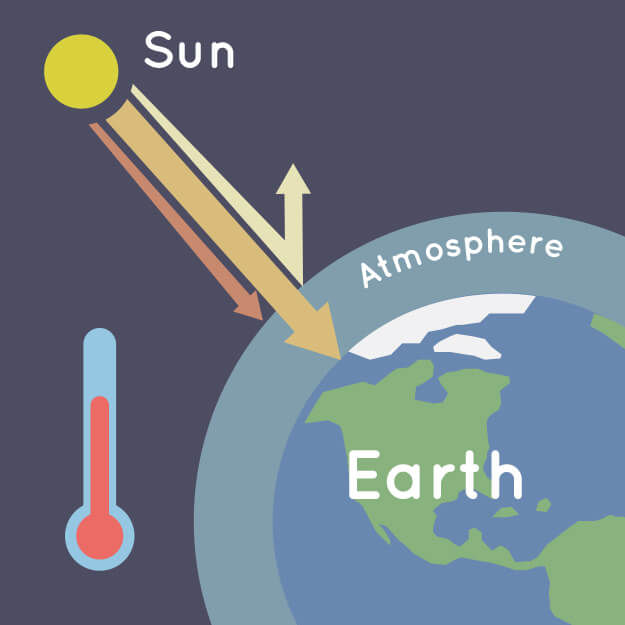 In a greenhouse, sunlight passes through the glass and warms the plants and air inside. The glass then traps the warm air, preventing it from escaping. Similarly, radiation emitted by objects inside the greenhouse cannot exit through the glass. This effect also explains why a car parked in the sun with its windows closed becomes excessively hot.
In a greenhouse, sunlight passes through the glass and warms the plants and air inside. The glass then traps the warm air, preventing it from escaping. Similarly, radiation emitted by objects inside the greenhouse cannot exit through the glass. This effect also explains why a car parked in the sun with its windows closed becomes excessively hot.
Kinetic Theory of Gases
According to it ideal gas particles are assumed to be tiny and collisions made by particles are perfectly elastic in nature.
Boyle’s law is pV = constant
where T = constant
Charle’s Law, V ∝ T
where p = constant , p ∝ T
when V = constant.
The ideal gas equation is pV = nRT
where, p, V , n, R and T are respectively pressure, volume, number of moles, gas constant and temperature of the gas.
|
528 videos|2108 docs|339 tests
|
FAQs on Heat and Thermodynamics - General Awareness for SSC CGL
| 1. What is the relationship between heat and temperature? |  |
| 2. How do different temperature scales relate to each other? |  |
| 3. What is thermal expansion and how does it relate to temperature? |  |
| 4. What is specific heat and how does it affect temperature changes? |  |
| 5. How does thermodynamics play a role in understanding heat and temperature changes? |  |





















