Hydration of Alkenes - Addition of H₂O by Oxymercuration | Chemistry Optional Notes for UPSC PDF Download
Hydration of Alkenes - Addition of H₂O by Oxymercuration
Water adds to alkenes to yield alcohols, a process called hydration. The reaction takes place on treatment of the alkene with water and a strong acid catalyst, such as H2SO4, by a mechanism similar to that of HX addition. Thus, as shown in Figure 8.4, protonation of an alkene double bond yields a carbocation intermediate, which reacts with water to yield a protonated alcohol product, ROH2+. Loss of H+ from this protonated alcohol gives the neutral alcohol and regenerates the acid catalyst.
Figure 8.4 MECHANISM Mechanism of the acid-catalyzed hydration of an alkene to yield an alcohol. Protonation of the alkene gives a carbocation intermediate, which reacts with water. The initial product is then deprotonated.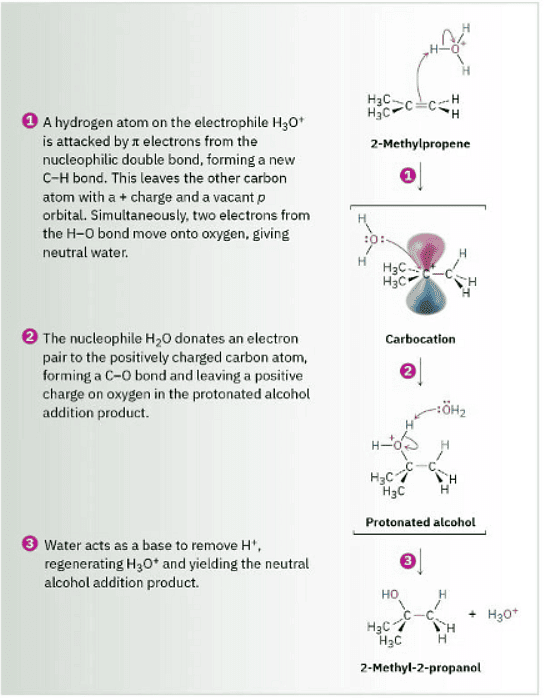
Most ethanol throughout the world is now made by fermentation of biological precursors, such as corn and sugar, but acid-catalyzed alkene hydration is particularly suited to large-scale industrial procedures, and approximately 90,000 tons of ethanol is manufactured each year in the United States by hydration of ethylene. The reaction is of little value in the laboratory, however, because it requires high temperatures—250 °C in the case of ethylene—and strongly acidic conditions.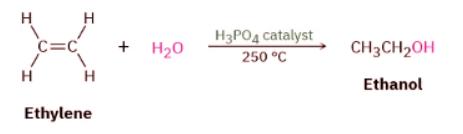
Acid-catalyzed hydration of double bonds is also uncommon in biological pathways. Instead, biological hydrations usually require that the double bond be adjacent to a carbonyl group for reaction to proceed. Fumarate, for instance, is hydrated to give malate as one step in the citric acid cycle of food metabolism. Note that the requirement for an adjacent carbonyl group in the addition of water is the same for the elimination of water. But will note for now that the reaction is not an electrophilic addition but instead occurs through a mechanism that involves formation of an anion intermediate followed by protonation by an acid HA.
When it comes to circumventing problems like those with acid-catalyzed alkene hydrations, laboratory chemists have a great advantage over the cellular “chemists” in living organisms. Laboratory chemists are not constrained to carry out their reactions in water solution; they can choose from any of a large number of solvents. Laboratory reactions don’t need to be carried out at a fixed temperature; they can take place over a wide range of temperatures. And laboratory reagents aren’t limited to containing carbon, oxygen, nitrogen, and a few other elements; they can contain any element in the periodic table.
In the laboratory, alkenes are often hydrated by the oxymercuration–demercuration procedure, which involves electrophilic addition of Hg2+ to the alkene on reaction with mercury(II) acetate [(CH3CO2)2Hg. The intermediate organomercury compound is then treated with sodium borohydride, NaBH4, and demercuration occurs to produce an alcohol. For example: Alkene oxymercuration is closely analogous to halohydrin formation. The reaction is initiated by electrophilic addition of Hg2+ (mercuric) ion to the alkene to give an intermediate mercurinium ion, whose structure resembles that of a bromonium ion (Figure 8.5). Nucleophilic addition of water as in halohydrin formation, followed by the loss of a proton, then yields a stable organomercury product. The final step, demercuration of the organomercury compound by reaction with sodium borohydride, is complex and involves radicals. Note that the regiochemistry of the reaction corresponds to Markovnikov addition of water; that is, the −OH group attaches to the more highly substituted carbon atom, and the −H attaches to the less highly substituted carbon. The hydrogen that replaces mercury in the demercuration step can attach from either side of the molecule depending on the exact circumstances.
Alkene oxymercuration is closely analogous to halohydrin formation. The reaction is initiated by electrophilic addition of Hg2+ (mercuric) ion to the alkene to give an intermediate mercurinium ion, whose structure resembles that of a bromonium ion (Figure 8.5). Nucleophilic addition of water as in halohydrin formation, followed by the loss of a proton, then yields a stable organomercury product. The final step, demercuration of the organomercury compound by reaction with sodium borohydride, is complex and involves radicals. Note that the regiochemistry of the reaction corresponds to Markovnikov addition of water; that is, the −OH group attaches to the more highly substituted carbon atom, and the −H attaches to the less highly substituted carbon. The hydrogen that replaces mercury in the demercuration step can attach from either side of the molecule depending on the exact circumstances.
Figure 8.5: Mechanism of the oxymercuration of an alkene to yield an alcohol. (1 ) Electrophilic addition of Hg2+ gives a mercurinium ion, which (2) reacts with water as in halohydrin formation. Loss of a proton gives an organomercury product, and (3) reaction with NaBH4 removes the mercury. The product of the reaction is a more highly substituted alcohol, corresponding to Markovnikov regiochemistry.
FAQs on Hydration of Alkenes - Addition of H₂O by Oxymercuration - Chemistry Optional Notes for UPSC
| 1. What is oxymercuration and how does it relate to the hydration of alkenes? |  |
| 2. How does oxymercuration differ from other methods of alkene hydration? |  |
| 3. What is the role of mercury in the oxymercuration reaction? |  |
| 4. Can you explain the mechanism of oxymercuration in detail? |  |
| 5. What are the advantages of using oxymercuration for alkene hydration? |  |

|
Explore Courses for UPSC exam
|

|
















