Important Points: Structure of the Atom | Science Class 9 PDF Download
| Table of contents |

|
| Introduction |

|
| Historical Perspective |

|
| Electron Distribution Rules |

|
| Valency |

|
| Isotopes |

|
| Isobars |

|
| Practice Questions |

|
Introduction
The evolution of atomic models unfolds a compelling narrative in scientific history. Beginning with Dalton's assumption of an indivisible atom, subsequent discoveries by Goldstein, Thomson, and Chadwick reshaped our understanding. Thomson's plum pudding and Rutherford's nuclear models provided insights, while Bohr's model addressed some issues. Concepts such as electron distribution, valency, atomic, and mass numbers gained significance. The discovery of isotopes and isobars expanded our knowledge, finding applications in diverse fields, from nuclear reactors to medical sciences.
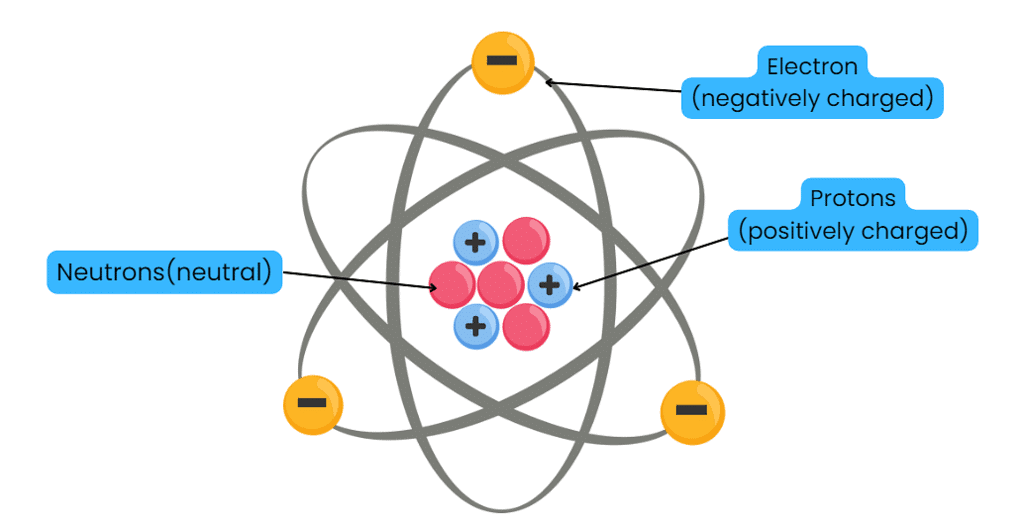
Historical Perspective
 Discoveries in Atomic Theory
Discoveries in Atomic Theory
- Dalton's Assumption (1803): Atom is indivisible.
- Goldstein's Discovery (1866): Discovered canal rays, leading to the identification of protons (positively charged particles).
- Thomson's Discovery (1897): Discovered electrons with a negative charge.
- Chadwick's Discovery: Identified neutrons with no charge.
Thomson's Model
- Electrons are distributed in the sphere of protons.
- Atom is electrically neutral.
- Known as the plum pudding model.
Rutherford's Model
- Experiment with α-particles on thin gold foil.
- Conclusions: Atom mostly empty space.
- +ve charge concentrated in a small volume (nucleus).
- Nuclear model: Nucleus (protons) at the center, electrons orbiting.
- Drawback: Electrons should lose energy and collapse, making atoms unstable.
Bohr's Model
- Electrons in discrete orbits (energy levels).
- Electrons in these orbits do not radiate energy.
- Drawbacks: Violates Heisenberg Uncertainty Principle, fails for larger atoms.
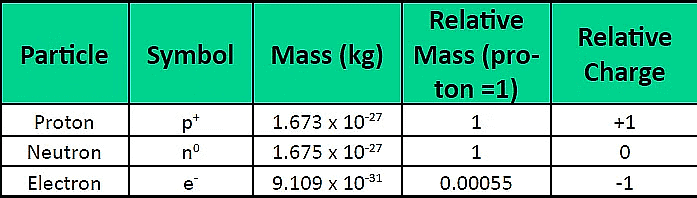 Atomic Structure
Atomic Structure
Discovery of Neutron
- In 1932, J. Chadwick made a groundbreaking discovery in the realm of atomic particles by identifying a subatomic particle with no charge and a mass nearly equivalent to that of a proton.
- This neutral particle, eventually named the neutron, is a constituent of the nucleus in all atoms, excluding hydrogen.
- Neutrons, denoted as 'n,' play a crucial role in determining the mass of an atom. The total mass of an atom is the sum of the masses of protons and neutrons residing within the nucleus.
Electron Distribution Rules
The distribution of electrons within different orbits of an atom was proposed by Bohr and Bury. The following rules guide the assignment of electron numbers to various energy levels or shells:
1. Maximum Electrons in a Shell: The maximum number of electrons present in a shell is determined by the formula 2n², where 'n' denotes the orbit number or energy level index (1, 2, 3, ...). This leads to specific maximum electron counts for various shells: 2 in the first orbit (K-shell), 8 in the second orbit (L-shell), 18 in the third orbit (M-shell), 32 in the fourth orbit (N-shell), and so forth.

2. Maximum Electrons in Outermost Orbit: The outermost orbit can accommodate a maximum of 8 electrons.
3. Step-wise Filling of Shells: Electrons are allocated to shells in a step-wise manner, ensuring that inner shells are filled before accommodating electrons in a given shell.
Valency
- Valency refers to the combining capacity of an atom, indicating its tendency to react with other atoms and form molecules.
- This property is associated with the arrangement of electrons in the outermost shell of an atom.
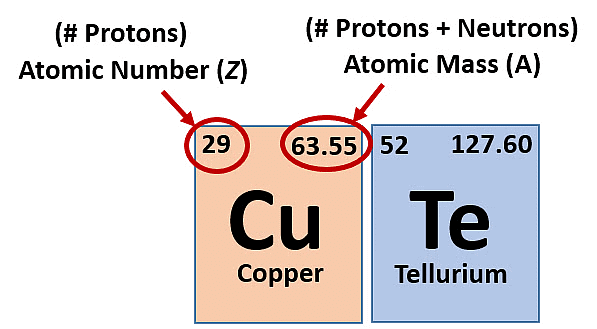
Atomic Number (Z)
- Total protons in an atom's nucleus.
- Represents the element.
Mass Number (A)
- Protons + Neutrons.
- Element representation: AXZ.
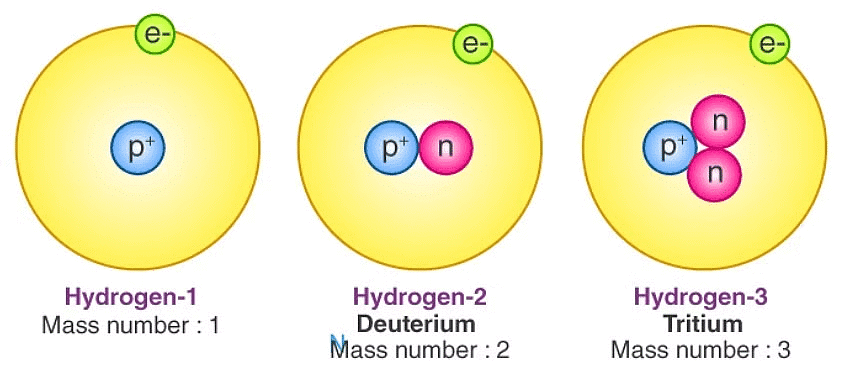
Isotopes
- Atoms of the same element with different mass numbers.
- Same chemical properties, different physical properties.
- Applications in nuclear reactors, cancer treatment, and medical fields.
Isobars
- Atoms of different elements with the same mass number.
- Different chemical properties.

Practice Questions
Ques. Is it possible for the atom of an element to have one electron, one proton and no neutron? If so, name the element.
Ans. Yes, it is true for the hydrogen atom which is represented as 1H1 . It is having one electron, one proton and no neutron.
Ques. What do you understand by the ground state of an atom?
Ans. The state of an atom where all the electrons in the atom are in their lowest energy levels is called the ground state.
Ques. Who identified the sub-atomic particle electron?
Ans. J.J. Thomson discovered the sub-atomic particle electron and proved that it existed without ever being able to see or isolate one.
Ques. Who discovered the nucleus of the atom?
Ans. Rutherford and his co-workers performed alpha-particle scattering experiments which led to the discovery of the atomic nucleus of atom.
|
88 videos|369 docs|67 tests
|
FAQs on Important Points: Structure of the Atom - Science Class 9
| 1. What is the historical perspective of atomic theory? |  |
| 2. What are the rules for electron distribution in an atom? |  |
| 3. What is valency and how is it determined? |  |
| 4. What are isotopes and how do they differ from each other? |  |
| 5. What are isobars and what distinguishes them from isotopes? |  |

|
Explore Courses for Class 9 exam
|

|



















