Introduction to Catalysis - Chemical Engineering PDF Download
Introduction to catalysis
The science and technology of catalysis is of great significance as it affects our daily life. Four major sectors of the world economy; petroleum and energy production, chemicals and polymer production, food industry and pollution control, involve catalytic processes.

Fig. 1. Four major sectors of world economy that involves catalytic processes
Catalysts are used to produce f uels such as gasoline, diesel, heating oil, fuel oil etc. Production of plastics, synthetic rubbers, fabrics, cosmetics etc. involve catalytic processes. The production of clean energy from renewable energy sources, such as hydrogen for fuel cells and transportation fuels from non-edible biomass are also catalyst depend ent processes. Automobile emission catalysts are used to reduce emissions of CO, NOx and hydrocarbon s from mobile vehicles. Catalysts are also used in the production of the polymers including adhesives, coatings, foams, textile and industrial fibers. The pharmaceutical industry uses catalysts for production of drugs that are used to save lives and improve the health of people. Catalysts are also widely used in food processing. More than 90 % of industrial processes actually use catalysts in one form or the other. Owing to expanding need of mankind, production in all sectors is increasing at a fast rate and catalysis science and technology has a major contribution in this. Thrusts are being given in the areas of catalyst upgrading to new and more efficient catalysts. Increasing catalyst life is another area of importance to maximize catalyst efficiency.
History : Catalyst technology has been used for many centuries. It ranged from inorganic catalyst to make soaps to enzyme catalysts for producing wines, cheese and other food and beverages. The industrial catalyst technology started with the large-scale production of sulfuric acid on platinum catalyst in 1875. In subsequent years, various major catalytic processes were invented. In 1903, ammonia oxidation on Pt gauge was developed by Ostwald for nitric acid production. Another major breakthrough was ammonia synthesis with promoted iron in 1908- 1914 by Mittasch, Bosch and Haber. Conversion of synthesis gas to liquid hydrocarbons by hydrogenation of CO, which was developed in 1920-1940, was a major development in the energy sector. In petroleum industry, the development of catalytic cracking process during 1935- 1940 changed the energy scenario. This process used a solid catalyst in the petroleum industry for the first time. Subsequent decades saw the development of various catalytic hydrocarbon processes such as catalytic naphtha reforming (1950) and hydrotreating for removal of sulphur, nitrogen, metals from petroleum feed stock (1960). With the discovery of Ziegler-Natta catalyst in 1955, the polymer industry grew significantly. The first large scale industrial homogeneous catalytic process came up in 1960 in the form of Walker process for making acetaldehyde from ethylene. The development of shape selective catalysts such as molecular sieves or zeolites for cracking (1964) resulted in the production of exclusively shape selective products. The other major development in catalysis was in 1970-1980 for environmental pollution control. Noble metal catalysts were developed for emission control of CO, NOx and hydrocarbons from automobiles. Vanadia-titania and zeolite catalysts were developed for selective reduction of NOx. Catalysis is a continuously growing area and discovery of new catalysts and their applications has led to major development in the chemical industry. The economic significance of the catalyst industry is enormous. The catalytic processes contribute greater than 35% of global GDP. The world catalyst industry amounts to US $ 12 billion. It is expected to grow annually by 6 % to US $16 billion US$ in 2012. Polymerization catalysts are expected to grow most rapidly due to significant expansion in polymer industry. Enzyme and organometallic catalysts will also grow. Reduction of sulphur levels in fuels and ongoing shifts towards heavy grade crude oil with high sulphur content is expected to contribute to the growth of catalytic hydrocarbon industry.
Catalysis involves understanding of the thermodynamics, kinetics, electronic interaction, crystal structure, reactor design and process development for a catalytic process. It is an interdisciplinary area involving contribution from chemical engineers, chemists and material scientists for successful implementation of the entire process starting from preparation of catalysts to final utilization in a chemical reactor.
Catalytic reactions In a thermodynamically feasible chemical reaction, when addition of a small amount a chemical substance increases the rate of attainment of chemical equilibrium but the substance itself does not undergo any chemical change, then the reaction is called a catalytic reaction. The substance that enhances the reaction rate is called a catalyst. Catalysts work by providing alternative mechanism involving a different transition state of lower energy. Thereby, the activation energy of the catalytic reaction is lowered compared to the uncatalyzed reaction as shown in Fig 2.

Fig. 2 . Comparison of activation energies of exothermic catalytic and non-catalytic reactions
A catalyst accelerates both the rates of the forward and reverse reaction. Equilibrium of a reversible reaction is not altered by the presence of the catalyst. For example, when oxidation of SO2 is carried out in the presence of three different catalysts, namely Pt, Fe2O3 and V2O5 , the equilibrium composition is the same in all three cases. Another important characteristic of catalyst is its effect on selectivity. The presence of different catalysts can result in different product distribution from the same starting material. For example, decomposition of ethanol in the presence of different catalysts results in different products as shown below.
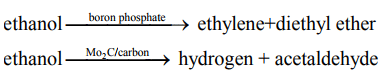
Types of catalytic reactions
Catalytic reactions can be divided into two main types –
1. Heterogeneous
2. Homogeneous
Heterogeneous catalysis
In heterogeneous catalytic reaction, the catalyst and the reactants are in different phases. Reactions of liquid or gases in the presence of solid catalysts are the typical examples. An example is the Contact Process for manufacturing sulphuric acid, in which the sulphur dioxide and oxygen are passed over a solid vanadium oxide catalyst producing sulphur trioxide. Several hydrocarbon transformation reactions such as cracking, reforming,dehydrogenation, isomerization also fall in this category.
Homogeneous catalysis
In a homogeneous catalytic reaction, the catalyst is in the same phase as the reactants. Typically, all the reactants and catalysts are either in one single liquid phase or gas phase. Most industrial homogeneous catalytic processes are carried out in liquid phase. Ester hydrolysis involving general acid-base catalysts, polyethylene production withorganometallic catalysts and enzyme catalyzed processes are some of the important examples of industrial homogeneous catalytic processes.
Relative significance
Catalytic processes have great significance and about 90 % of all chemical industry involves catalytic processes. Of all the industrial catalytic processes, approximately 80 % involve the use of solid catalysts, 17 % homogeneous catalysts and rest 3 % biocatalysts. Thus, heterogeneous catalysts, particularly solid catalysts, dominate the industrial catalytic processes. Though the contributions of homogeneous catalytic processes in chemical industry are significantly smaller than that of heterogeneous catalytic processes, but because of high selectivities, homogeneous process are finding increasing importance for production of many important value added products such as manufacturing of tailor made plastics, fine chemicals, pharmaceutical intermediates etc.
Heterogeneous catalytic theory
In general, it is believed that the entire surface of the solid catalyst is not responsible for catalyzing any reaction. Only certain sites on the catalyst surface actually participate in the reaction and these sites are called active sites on the catalysts. These sites may be the unsaturated atoms resulting from surface irregularities or atoms with chemical properties that enable the interaction with the adsorbed reactant atoms or molecules. Activity of the catalyst is directly proportional to the number of these active sites available on the surface and is often expressed in terms of turnover frequency. Turnover frequency is defined as the number of molecules reacting per active site per second at the condition of experiments. A solid catalytic reaction A → B goes through the following steps. The steps are illustrated inFig 3.
1.Transportation of reactant (A) from bulk fluid to pore mouth on the external surface of catalysts pellets
2. Diffusion of the reactant (A) from the pore mouth through the catalyst pores to the immediate vicinity of internal catalytic surface
3. Adsorption of reactant (A) onto the catalyst surface
4. Reaction of (A) on the catalyst surface producing product (B)
5. Desorption of the product (B) from the surface
6. Diffusion of the product (B) from interior part of the pores to the pore mouth on the external surface
7. Transfer of the product (B) from pore mouth on the external surface to the bulk fluid

Fig. 3. Steps in solid catalytic reactions
The overall rate of reaction is equal to the rate of slowest step in the mechanism. When the mass transfer and diffusion steps [1,2,6,7] are very fast compared to adsorption and reaction steps [3,4,5], concentration in the immediate vicinity of the active sites is the same or indistinguishable from that in the bulk fluid. Consequently, the transport or diffusion steps do not affect the overall rate of the reaction. Alternatively, if reaction and diffusion steps are fast compared to the mass transfer steps, then mass transfer does affect the rate of reaction. When mass transfer from the bulk phase to the pore mouth is slow and affects the reaction rate, then changing the flow conditions past the catalyst should change the overall reaction rate. In case of porous catalysts, diffusion within the catalyst pores may limit the reaction rate. Underr this condition external flowdoes not affect the reaction rate but internal diffusion does affect.
FAQs on Introduction to Catalysis - Chemical Engineering
| 1. What is catalysis in chemical engineering? |  |
| 2. How does catalysis impact chemical reactions? |  |
| 3. What are the different types of catalysts used in catalysis? |  |
| 4. How are catalysts chosen for a specific reaction? |  |
| 5. What are the challenges in catalysis research and development? |  |

|
Explore Courses for Chemical Engineering exam
|

|

















