Ionic Compounds | Chemistry for Grade 10 PDF Download
Forming ions
An ion is an atom or group of atoms with a positive or negative charge. Ions form when atoms lose or gain electrons to obtain a full outer shell:
- metal atoms lose electrons to form positively charged ions
- non-metal atoms gain electrons to form negatively charged ions
Forming positive ions
- Metal atoms lose electrons from their outer shell when they form ions:
- the ions are positive, because they have more protons than electrons
- the ions formed have full outer shells
- the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell
- For elements in groups 1, 2 and 3, the number of electrons lost is the same as the group number.

A sodium atom loses one electron to form a sodium ion
Forming negative ions
- The outer shells of non-metal atoms gain electrons when they form ions:
- the ions formed are negative, because they have more electrons than protons
- the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell
- For elements in groups 6 and 7, the charge on the ion is equal to (8 minus group number).

An oxygen atom gains two electrons to form an oxide ion
- Ions are formed by the transfer of electrons.
Example of ion charges and groups
Example: Sulfur is in group 6 of the periodic table. What is the charge on its ions, and is the charge positive or negative?
The charge is negative, since sulfur is a non-metal. The charge on the ion is (8 - 6) = 2.
Forming ionic bonds
Positive and negative ions form when a metal reacts with a non-metal, by transferring electrons. The oppositely charged ions are strongly attracted to each other, forming ionic bonds.
Dot and cross diagrams
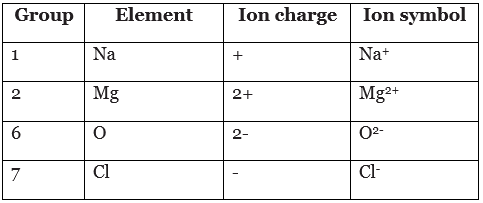
- A dot and cross diagram models the transfer of electrons from metal atoms to non-metal atoms. The electrons from one atom are shown as dots, and the electrons from the other atom are shown as crosses. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The diagrams show two ways of representing this electron transfer.

The outer electron from a sodium atom transfers to the outer shell of a chlorine atom
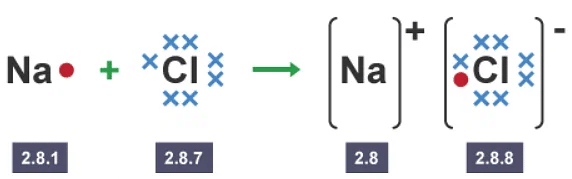
The electron from a sodium atom transfers to a chlorine atom
Modelling ionic bonding
- The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride.
1. Ionic bonding in sodium chloride
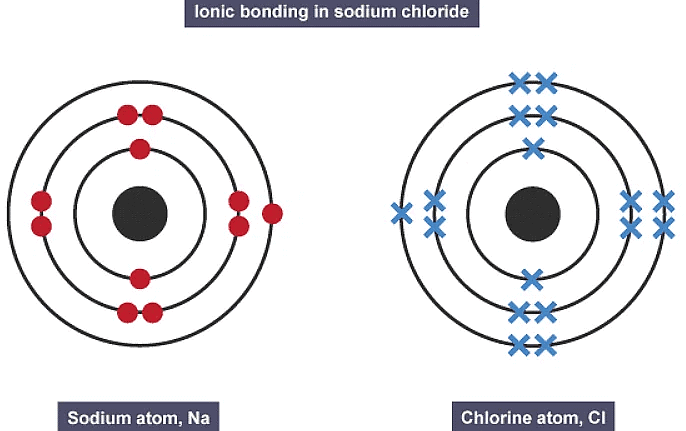
2. Ionic bonding in sodium chloride

3. Ionic bonding in magnesium oxide

Example: Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements.
The ionic lattice
An ionic compound is a giant structure of ions. The ions have a regular, repeating arrangement called an ionic lattice. The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other.
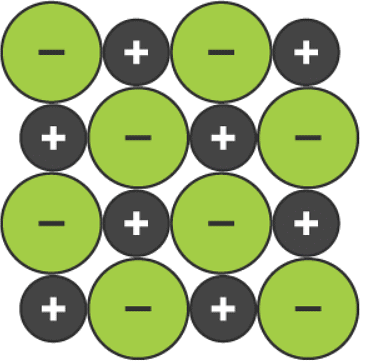
A two-dimensional space-filling model for the ionic lattice in sodium chloride
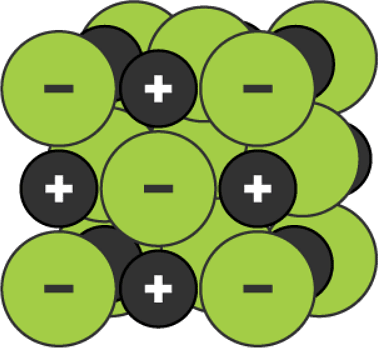
A three-dimensional space-filling model for the ionic lattice in sodium chloride
Remember that the lattice arrangement is giant - for example, a single grain of salt may contain 1.2 × 1018 (1,200,000,000,000,000,000) ions. The lattice arrangement continues in three dimensions. This is why solid ionic compounds form crystals with regular shapes.

A three-dimensional ball and stick model for the ionic lattice in sodium chloride
Ionic bonding
- An ionic lattice is held together by strong electrostatic forces of attraction between the oppositely charged ions. The forces act in all directions in the lattice. This is called ionic bonding.
Representing ionic compounds
- Different types of model are used to represent giant ionic structures. Each has its advantages and limitations. For example:
- the two-dimensional space-filling model clearly shows the arrangement of ions in one layer, but it does not show how the next layer of ions is arranged
- the three-dimensional ball and stick model shows the arrangement of ions in a larger section of the crystal, but using sticks for bonds is misleading because the forces of attraction between ions actually act in all directions
- the three-dimensional model is also misleading because it shows lots of free space between the ions, which there isn't
Properties of ionic compounds
- Ionic compounds have regular structures, called giant ionic lattices. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions between the oppositely charged ions. The structure and bonding of ionic compounds explain their properties.
High melting points and boiling points
Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature.
- Energy must be transferred to a substance to make it melt or boil. This energy overcomes the strong electrostatic forces of attraction which act in all directions between the oppositely charged ions:
- some forces are overcome during melting
- all remaining forces are overcome during boiling
- The more energy needed, the higher the melting point or boiling point. Since the electrostatic forces of attraction between oppositely charged ions are strong, their melting and boiling points are high.
Explanation
- Ionic compounds are held together by electrostatic forces between the oppositely charged ions. These forces are usually referred to as ionic bonding. As the ionic lattice contains such a large number of ions, a lot of energy is needed to overcome this ionic bonding so ionic compounds have high melting and boiling points.
- The strength of the ionic bonds depends on the charge on the ions. Ions with higher charge will have stronger forces between them, so will need more energy in order to overcome these forces.

Ionic bonds between Mg2+ and O2- ions are stronger than those between Na+ and Cl- ions
Conducting electricity
- A substance can conduct electricity if:
- it contains charged particles, such as ions, and
- these particles are free to move from place to place
- An ionic compound can conduct electricity when:
- it has melted to form a liquid, or
- it has dissolved in water to form an aqueous solution
- Both these processes allow ions to move from place to place. Ionic compounds cannot conduct electricity in the solid state because their ions are held in fixed positions and cannot move.
Ionic compounds conduct electricity when melted or in solution. They are insulators when solid.
|
78 videos|87 docs|11 tests
|



















