Modern Physics | Physics for JAMB PDF Download
Modern Physics
1. Nature of light
It was a matter of great interest for scientists of know that what exactly from the light is made up of or how the light behaves. This is briefly described over here
1.1 Newton's Corpuscular theory :
Newton was the first scientist who said that light is made 0 up tiny elastic particles called "Corpuscles" which travels with the velocity of light. So according to Newtons, light is a particle.
1.2 Huygen's wave theory :
Huygen was a scientist working parallel to Newton who come with a drastically different idea for nature of light & said that light is not a particle but a wave.
1.3 Maxwell's electromagnetic wave theory :
During the time of Huygen, his views regarding nature of light were not accepted as newton was a popular scientist of his time. but, when maxwell asserted that light is a electromagnetic wave, scientists started believing that light is a wave.
1.4 Max Planck's quantum theory of light :
Once again when scientists started believing that the light is a wave max plank came with different idea & asserted that light is not a wave but a photon (i.e. a particle) which he proved through black body radiation spectrum. At this time there was a great confusion about the nature of light which was solved by de-broglie from where origin of theory of matter wave come into picture.
1.5 Debroglie Hypothesis
It supports dual nature of light (wave nature and particle nature). According to him the light consists of particles associated with definite amount of energy and momentum. These particles were later named as photons.
The photon posses momentum and is given by
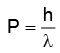 ...(1)
...(1)
P = momentum of one photon
λ = wavelength of wave.
h = Plank's constant = 6.62 × 10-34 Js.
A photon is a packet of energy. It posses energy given by
...(2)
where c = speed of light
Debroglie relates particle property (momentum) with wave property (wavelength) i.e. he favours dual nature of light.
Electron volt : It is the energy gained by an electron when it is accelerated through a potential difference of one volt.
1 eV = 1.6 × 10-19 Joule.
Now from eq. (2)
in Joule.
where λ is in Å
Properties of Photon :
1. Photon travels with speed of light.
2. The rest mass of a photon is zero.
3. There is no concept of photon conservation.
4. All the photons of a particular frequency or wavelength posses the same energy irrespective of the intensity of the radiation.
5. The increase in the intensity of the radiation imply an increase in the number of photon's crossing a given area per second.
- When light travels from one medium to another medium then
frequency = const (because it is the property of source)
but v, λ changes
Ex.1 A beam of light having wavelength l and intensity 1 falls normally on an area A of a clean surface then find out the number of photon incident on the surface.
Sol. Total energy incident in time t = I A t
Energy of one photon E =
Then number of photon incident in time t
|
260 videos|253 docs|230 tests
|
FAQs on Modern Physics - Physics for JAMB
| 1. What is modern physics? |  |
| 2. How does modern physics differ from classical physics? |  |
| 3. What are the key principles of quantum mechanics? |  |
| 4. How does relativity theory impact modern physics? |  |
| 5. What are the current frontiers of modern physics research? |  |

















