Monosubstituted cyclohexane- Stereochemistry PDF Download
1. When methyl group is axial it is sufficiently closer to the Syn axial Hydrogen’s to undergo 1,3-diaxial interactions and is repelled by them.

2. This 1, 3-diaxial interaction is similar to that in gauche conformation of butane. The axial methyl group in methyl cyclohexane is thus gauche to two ring carbons and when in equatorial positions it is anti to same nuclei.

3. When methyl group is equatorial, there are no 1, 3-diaxial interaction.
4. Equatorial methyl group don’t show any gauche butane like interactions. Monosubstituted cyclohexane exists in two non-equivalent diastereomeric chair conformations.

(I) is less stable than (ii) because of the presence of 1, 3-diaxial interactions.
1,2-Disubstituted cyclohexane


- Stability: A > C=D > B
- All 1, 2-disubstitutedcyclohexanes are achiral due to presence of plane of symmetry and two fold rotational axis hence all are optically inactive.
1, 3-disubstitutedcyclohexane


- Stability: - A>C=B>D
- 1,3-dimethylcyclohexane has two chiral centers, and can have four stereoisomers (22=4). Actually there are only three, the cis-1, 3-dimethylcyclohexane has a plane of symmetry and is achiral. Trans isomer has a twofold rotational axis hence it is also achiral. If different substituents are present all will be chiral.
1, 4-disubstututedcyclohexane


- Stability: - A > C = D > B
- In 1, 4-dimethylcyclohexane it does not have any chiral centre. It exists as Cis and Trans Diastereomers. Neither Cis nor Trans form is chiral because both have a plane of symmetry
Factors affecting stability of cyclohexane derivatives
1. Steric strain
2. Torsional strain
3. Dipole moment:- If μ> 0 , molecule is less stable, if μ=0, comparably more stable. For example, 1,2-diaxial is more stable than 1,2-diequatorial due to dipole-dipole repulsions.

4. Hydrogen bonding:- In cases where hydrogen bonding is possible, gauche form is more stable than the staggered form as in case of ethylene glycol.

Stable conformations of cyclohexandiols
1. 1,4-diol

2. 1,2-diol

3. 1,3-diol

Decalins- bicyclo[4,4,0]decane

Trans- decalin
Trans decalin is obtained by fusion between two equatorial bonds of cyclohexanes with 4 carbon system. In this two hydrogen atoms on the bridge head carbon are opposite to each other. Achiral, more stable due to diequatorial type structure.

Cis-decalin
When one axial and one equatorial bond of cyclohexanes ring are used for fusing 4 carbon chain system a decalin molecule results which has two hydrogen on the same side of bridgehead carbon.

(i) Less stable due to non-bonding interactions
(ii) No plane of symmetry
(iii) Molecule is chiral.
Note: Cis- and trans- isomers are conformational isomers. Energy of trans decalin is 25 kj/mol lesser than cis decalins.
If 1 position of decalin is substituted by Me- group then stability is reversed.


Fischer projection (2-D representation)
- Horizontal bonds- Points towards the observer
- Vertical bonds- Points away from the observer

Some important rules: In order to find out whether the two structures are identical or not, these projections can be manipulated only in specific ways. The following rules must be obeyed.
1. For comparison, a fischer projection may be rotated 180° in the plane of the paper & molecule remains same.

2. One interchange in the fischer projection leads to the enantiomers. Configuration at the
stereocenter changes from (S) to (R) and vice-versa.

3. Two or any even number of interchanges of the groups at the chiral centre, don’t changes the configuration.

4. A 900 rotation of the fischer projection formula about the chiral centre inverts the configuration.

5. It is not permitted lift projection formulae out of the plane of the paper and flip them over or view them from the opposite side of the paper. These operations, if done, are the same as breaking a bond to change the configuration of the original molecule.
For Example:

6. Fischer projection can be manipulated by rotating a group of any three ligands in a clockwise or anticlockwise direction; the fourth ligand doesn’t change its position (Such a manipulation is equivalent to two interchanges).

Flying–wedge representation (3-D representation)
- Solid wedge (thick line)- bond above the plane of paper
- Broken wedge (dashed line) – a bond below the plane of the paper
- Continuous lines (solid lines)- bonds in the plane of the paper
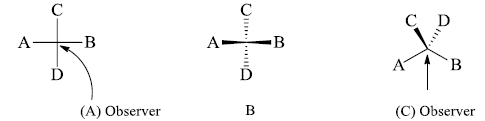
Fischer projection into flying wedge and vice-versa
We draw the bonds which are towards the observer by solid wedge and the bonds away from observer by dashed lines. Then the above shown molecule becomes.

We want to convert this molecule into flying wedge projection formula for this hold the atom C and D and starts the process of bending so that a inverted V is formed i.e.^. This inverted v represents the atoms in one plane. Now draw the B below this plane which is shown below in this formula .The atom shown above is represented by above the plane in flying wedge formula.
We are observing from the bottom of the right side. If we will observe from top of this molecule then we find that

Realistic representation of stereo structures of molecules
1. Sawhorse representation:
The sawhorse formula indicates the spatial arrangement of all the groups
on two adjacent carbon atoms is represented by a diagonal line usually from lower left to upper right. The left handed bottom end represents the atom nearest to the observer and the right hand top end represents the atom away from the observer. Two of the remaining bonds to the two atoms are drawn vertically and other four at +1200 and-1200 angles.

2. Newman projection: The Newman projection formula is planar projection formula of the sawhorse formula. Newman projection is similar to the sawhorse projection, but represents the spatial arrangements of all the groups on the two carbon atoms. Here, a molecule is viewed along the axis of a carbon –carbon bond. The carbon towards the front is represented by a dot (.) and the carbon towards the rear by a circle.

Conversion of fischer projection into sawhorse and newman formula
When we observe the molecule from the bottom of the right side, the bond between the adjacent carbon atoms is represented by a diagonal line usually from the lower left to the upper right. The left handed bottom end represents the atom nearest to the observer and the right hand top end represents the atom away from the observer.
If we want to convert eclipsed form of flying wedge projection into staggered form then hold C2 atom in hand in its position and rotate the C3 atom by 1800 . The atoms which are towards the observer are away from the observer and the atoms which are away from the observer becomes towards the observer.
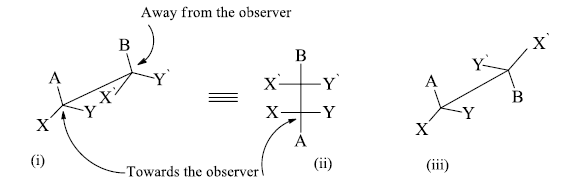
i. Eclipsed conformation (Flying wedge projection).
ii. Eclipsed conformation (Fischer projection).
iii. Staggered conformation (Flying wedge projection).
FAQs on Monosubstituted cyclohexane- Stereochemistry
| 1. What is a monosubstituted cyclohexane? |  |
| 2. What is the stereochemistry of monosubstituted cyclohexane? |  |
| 3. How does the stereochemistry of a monosubstituted cyclohexane affect its physical properties? |  |
| 4. How can the stereochemistry of a monosubstituted cyclohexane be determined experimentally? |  |
| 5. What are some common examples of monosubstituted cyclohexanes? |  |

|
Explore Courses for Chemistry exam
|

|

















