NCERT Exemplar: Hydrogen (Old NCERT) | Chemistry for Grade 11 PDF Download
MULTIPLE CHOICE QUESTIONS - I
Q.1. Hydrogen resembles halogens in many respects for which several factors are responsible. Of the following factors which one is most important in this respect?
(1) Its tendency to lose an electron to form a cation.
(2) Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
(3) Its low negative electron gain enthalpy value.
(4) Its small size.
Ans. (2)
Solution.
Halogens have the tendency to gain one electron and acquire inert gas configuration. Hydrogen also accepts one electron and acquires helium configuration.

Q.2. Why does H+ ion always get associated with other atoms or molecules?
(1) Ionisation enthalpy of hydrogen resembles that of alkali metals.
(2) Its reactivity is similar to halogens.
(3) It resembles both alkali metals and halogens.
(4) Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to small size it cannot exist free.
Ans. (4)
Solution.
H→H+ +e–
H+ has a very small size (~1.5 x 10-3 pm) compared to normal atomic and ionic sizes of 50 to 220 pm. It does not exist freely and is always associated with other atoms or molecules.
Q.3. Metal hydrides are ionic, covalent or molecular in nature. Among LiH, NaH, KH, RbH, CsH, the correct order of increasing ionic character is
(1) LiH > NaH > CsH > KH>RbH
(2) LiH < NaH < KH < RbH < CsH
(3) RbH > CsH > NaH > KH > LiH
(4) NaH > CsH > RbH > LiH > KH
Ans. (2)
Solution.
Ionic character increases as the size of the atom increases.
LiH < NaH < KH < RbH < CsH
Q.4. Which of the following hydrides is electron-precise hydride?
(i) B2H6
(ii) NH3
(iii) H2O
(iv) CH4
Ans. (4)
Solution.
CH4 is an electron precise hydride since there are exact number of electrons to form normal covalent bonds.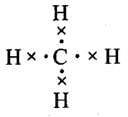
Q.5. Radioactive elements emit α, β and γ rays and are characterised by their half lives. The radioactive isotope of hydrogen is
(1) Protium
(2) Deuterium
(3) Tritium
(4) Hydronium
Ans. (3)
Solution.
Nucleides with n/p (neutron-proton) ratio > 1.5 are usually radioactive. For example, tritium (n = 2, p = 1).
Q.6. Consider the reactions
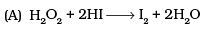

Which of the following statements is correct about H2O2 with reference to these reactions?Hydrogen perioxide is ________.
(1) An oxidising agent in both (A) and (B)
(2) An oxidising agent in (A) and reducing agent in (B)
(3) A reducing agent in (A) and oxidising agent in (B)
(4) A reducing agent in both (A) and (B)
Ans. (b)
Solution.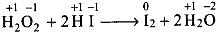
O.N. of oxygen is decreased from -1 (H2O2) to -2 (H2O), therefore, it is reduced and acts as an oxidizing agent.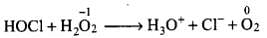
O.N. of oxygen is increased from -1 (H2O2) to O (O2), therefore, it is oxidized and acts as a reducing agent.
Q.7. The oxide that gives H2O2 on treatment with dilute H2SO4 is —
(i) PbO2
(ii) BaO2 .8H2O + O2
(iii) MnO2
(iv) TiO2
Ans. (b)
Solution.
Oxides such as BaO2, Na2O7, etc. which contain peroxide linkage (i.e., -O-O- or 022- ) on treatment with dil. H2SO4 give H2O2 but dioxides (O = M = O, where M is the metal atom) such as PbO2, MnO2, TiO2 do not give H2O2 on treatment with dil. H2SO4.
Q.8. Which of the following equations depict the oxidising nature of H2O2?
(i)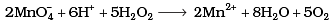
(ii)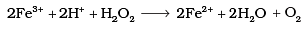
(iii)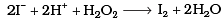
(iv)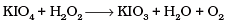
Ans. (c)
Solution.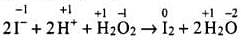
I- ions are oxidized to I2 (increase in O.N. from -1 to 0). Hence, H2O2 acts as an oxidizing agent.
Q.9. Which of the following equation depicts reducing nature of H2O2?
(i) 2[Fe(CN)6]4– + 2H+ + H2O2 → 2[Fe(CN)6]3– + 2H2O
(ii) I2 + H2O2 + 2OH– → 2I– + 2H2O + O2
(iii) Mn2+ + H2O2 → Mn4+ + 2OH–
(iv) PbS + 4H2O2 → PbSO4 + 4H2O
Ans. (b)
Solution.
I2 + H2O2 + 2OH–→ 2I– + 2H2O + O2
I2 is reduced to I–. Thus, H2O2 acts as a reducing agent.
Q.10. Hydrogen peroxide is ._________ .
(a) An oxidizing agent
(b) A reducing agent
(c) Both an oxidizing and a reducing agent
(d) Neither oxidizing nor reducing agent.
Ans. (c)
Solution.
H2O2 acts both as oxidizing and reducing agent.
Q.11. Which of the following reactions increases production of dihydrogen from synthesis gas?
(1)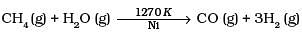
(2)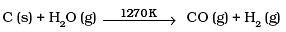
(3)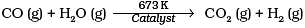
(4)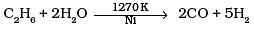
Ans. (c)
Solution.
To increase the production of H2 from synthesis gas, CO is oxidized to C02 by passing it over steam at 673 K in presence of a catalyst.
Thus, option (c) is correct.
Q.12. When sodium peroxide is treated with dilute sulphuric acid, we get ______.
(1) Sodium sulphate and water
(2) Sodium sulphate and oxygen
(3) Sodium sulphate, hydrogen and oxygen
(4) Sodium sulphate and hydrogen peroxide
Ans. (4)
Solution.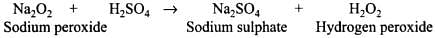
Q.13. Hydrogen peroxide is obtained by the electrolysis of ______.
(1) Water
(2) Sulphuric acid
(3) Hydrochloric acid
(4) Fused sodium peroxide
Ans. (b)
Solution.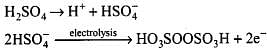

Q.14. Which of the following reactions is an example of use of water gas in the synthesis of other compounds?
(1)
(2)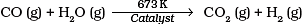
(3)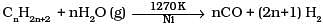
(4)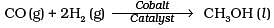
Ans. (4)
Solution.
Water gas is used in synthesis of compounds like methanol.
Q.15. Which of the following ions will cause hardness in water sample?
(1) Ca2+
(2) Na+
(3) Cl–
(4) K+
Ans. (1)
Solution.
Ca++ ions in the form Ca(HCO3)2 or CaCl2 or CaSO4 cause hardness in water, i.e., soluble salts of calcium can cause hardness in water.
Q.16. Which of the following compounds is used for water softening?
(i) Ca3(PO4)2
(ii) Na3PO4
(iii) Na6P6O18
(iv) Na2HPO
Ans. (c)
Solution.
(Sodium hexametaphosphate) commercially known as calgon is used for water softening.
2CaCl2 + Na2[Na4(P03)6] →Na2[Ca2(PO3)6] + 4NaCl
Q.17. Elements of which of the following group(s) of periodic table do not form hydrides.
(1) Groups 7, 8, 9
(2) Group 13
(3) Groups 15, 16, 17
(4) Group 14
Ans. (1)
Solution.
Group 7, 8, 9 elements do not form hydrides.
Q.18. Only one element of ________ forms hydride.
(1) Group 6
(2) Group 7
(3) Group 8
(4) Group 9
Ans. (1)
Solution.
Only one element chromium from group 6 forms hydride, (CrH).
MULTPLE CHOICE QUESTIONS - II
In the following questions two or more options may be correct.
Q.19. Which of the following statements are not true for hydrogen?
(1) It exists as diatomic molecule.
(2) It has one electron in the outermost shell.
(3) It can lose an electron to form a cation which can freely exist.
(4) It forms a large number of ionic compounds by losing an electron.
Ans. (c, d)
Solution.
It can lose an electron to form a cation which cannot freely exist. Generally, it does not form ionic compounds by losing an electron but forms a large number of covalent compounds by sharing electron.
Q.20. Dihydrogen can be prepared on commercial scale by different methods. In its preparation by the action of steam on hydrocarbons, a mixture of CO and H2 gas is formed. It is known as ____________.
(1) Water gas
(2) Syngas
(3) Producer gas
(4) Industrial gas
Ans. (a, b)
Solution.
A mixture of CO + H2 is known as water gas or syn gas (synthesis gas).
Q.21. Which of the following statement(s) is/are correct in the case of heavy water?
(1) Heavy water is used as a moderator in nuclear reactor.
(2) Heavy water is more effective as solvent than ordinary water.
(3) Heavy water is more associated than ordinary water.
(4) Heavy water has lower boiling point than ordinary water.
Ans. (1, 3)
Solution.
Heavy water is used as a moderator in nuclear reactor and is more associated than ordinary water.
Q.22. Which of the following statements about hydrogen are correct?
(1) Hydrogen has three isotopes of which protium is the most common.
(2) Hydrogen never acts as cation in ionic salts.
(3) Hydrogen ion, H+, exists freely in solution.
(4) Dihydrogen does not act as a reducing agent.
Ans. (1, 2)
Solution.
Among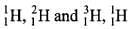 (protium) is most common. In ionic salts, hydrogen acts as anion (H-).
(protium) is most common. In ionic salts, hydrogen acts as anion (H-).
Q.23. Some of the properties of water are described below. Which of them is/are not correct?
(1) Water is known to be a universal solvent.
(2) Hydrogen bonding is present to a large extent in liquid water.
(3) There is no hydrogen bonding in the frozen state of water.
(4) Frozen water is heavier than liquid water.
Ans. (3, 4)
Solution.
There is extensive hydrogen bonding in ice. Ice is lighter than water due to empty spaces present in tetrahedrons formed by hydrogen bonds.
Q.24. Hardness of water may be temporary or permanent. Permanent hardness is due to the presence of
(i) Chlorides of Ca and Mg in water
(ii) Sulphates of Ca and Mg in water
(iii) Hydrogen carbonates of Ca and Mg in water
(iv) Carbonates of alkali metals in water
Ans. (1, 2)
Solution.
Permanent hardness of water is due to presence of following salts:
CaCl2; MgCl2; CaS04; MgS04
Q.25. Which of the following statements is correct?
(1) Elements of group 15 form electron deficient hydrides.
(2) All elements of group 14 form electron precise hydrides.
(3) Electron precise hydrides have tetrahedral geometries.
(4) Electron rich hydrides can act as Lewis acids.
Ans. (2, 3)
Solution.
All elements of group 14 form electron precise hydrides like CH4 which are tetrahedral in geometry.
Q.26. Which of the following statements is correct?
(i) Hydrides of group 13 act as Lewis acids.
(ii) Hydrides of group 14 are electron deficient hydrides.
(iii) Hydrides of group 14 act as Lewis acids.
(iv) Hydrides of group 15 act as Lewis bases.
Ans. (1, 4)
Solution.
All elements of group 13 will form electron deficient compounds which acts as Lewis acids.
All elements of group 14 will form electron precise compounds.
Electron rich hydrides have excess electrons which are present as lone pairs. Elements of group 15-17 form such compounds. NH3 has one lone pair, H2O has two lone pairs and HF has three lone pairs, and so these compounds act as Lewis bases.
Q.27. Which of the following statements is correct?
(1) Metallic hydrides are deficient of hydrogen.
(2) Metallic hydrides conduct heat and electricity.
(3) Ionic hydrides do not conduct electricity in solid state.
(4) Ionic hydrides are very good conductors of electricity in solid state.
Ans. (1, 2, 3)
Solution.
Metallic hydrides are non-stoichiometric hydrides. They conduct heat and electricity. Ionic hydrides conduct electricity only in molten or aqueous state.
SHORT ANSWER TYPE QUESTIONS
Q.28. How can production of hydrogen from water gas be increased by using water gas shift reaction?
Ans. Water gas is produced when superheated steam is passed over red hot coke or coal at 1270 K in the presence of nickel as catalyst.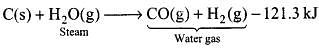
Pure H2 from water gas cannot be obtained easily because it is difficult to remove CO. Therefore, to increase the production of H2 from water gas, CO is oxidized to CO2 by mixing it with more steam and passing the mixture over heated FeCrO4 catalyst at 673 K.
The CO2 produced is removed by scrubbing with sodium arsenite solution.
Q.29. What are metallic/interstitial hydrides? How do they differ from molecular hydrides?
Ans. Metallic hydrides are formed by d- and f-:block elements. Their hydrides conduct heat and electricity. They are non-stoichiometric, being deficient in hydrogen. For example, LaH2.87, ybH2.55 etc.
| Metallic hydrides | Molecular hydrides |
| (1) These are formed by eland f-block elements. | (1) These are formed by p-block elements and some s-block elements (Be and Mg). |
| (2) They conduct electricity. | (2) They do not conduct electricity. |
| (3) They are hard and have metallic luster. | (3) They are volatile compounds having low melting and boiling points. |
Q.30. Name the classes of hydrides to which H2O, B2H6 and NaH belong.
Ans. H2O – Electron rich covalent hydride/molecular hydride
B2H6 – Electron deficient molecular hydride
NaH – Ionic hydride
Q.31. If same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Ans. The mass per unit volume (i.e., mass/volume) is called density. Since water expands on freezing, therefore, volume of ice for the same mass of water is more than liquid water. In other words, density of ice is lower than liquid water and hence ice floats on water.
Q.32. Complete the following equations:
(1)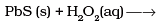
(2) 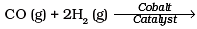
Solution.
Q.33. Give reasons:
(1) Lakes freeze from top towards bottom.
(2) Ice floats on water.
Ans. (1) During severe winter, the temperature of water in the lake keeps on decreasing. Since cold water is heavier, it keeps on going into the interior of the lake while warm water keeps on coming to the surface of the lake. This process continues till the temperature of entire water of lakes becomes 4°C. Since density of water is maximum at 277 K, any further decrease in the temperature will decrease its density. As a result, the temperature of the surface water keeps on decreasing and it ultimately freezes. Now, any further decrease in the temperature will decrease the temperature of water below 4°C. This process continues and as a result, the lakes keep on freezing from top to bottom.
(2) Density of ice is less than water due to presence of empty spaces created because of H-bonding between H2O molecules. Hence, ice floats on water.
Q.34. What do you understand by the term ‘auto protolysis of water’? What is its significance?
Ans. Autoprotolysis is a reaction in which two same molecules react to give ions with proton transfer. Water undergoes autoproteolysis, i.e., a proton from one molecule is transferred to another molecule.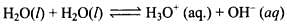

Q.35. Discuss briefly de-mineralisation of water by ion exchange resin.
Ans. Demineralised water free from all soluble mineral salts is obtained by passing water successively through a cation exchange and an anion exchange resin.
In cation exchange process, H+ exchanges for Na+, Ca2+, Mg2+ and in the anion exchange process OH exchanges for anions like CH, HCO3, S02-4, etc. H+ and OH– released combine to form water.
H+ + OH– → H2O
Q.36. Molecular hydrides are classified as electron deficient, electron precise and electron rich compounds. Explain each type with two examples.
Ans. Covalent or molecular hydrides are classified into three categories:
(i) Electron deficient hydrides: These hydrides do not have sufficient number of electrons to form normal covalent bonds. Examples are the hydrides of group 13 such as B2H6, (AlH3)n etc.
(ii) Electron precise hydrides: These have exact number of electrons to form normal covalent bonds. Examples are the hydrides of group 14 such as CH4, SiH4, etc.
(iii) Electron rich hydrides: These have more number of electrons than normal covalent bonds. The excess electrons are present in the form of lone pairs. Examples are the hydrides of group 15, 16 and 17 such as
Q.37. How is heavy water prepared? Compare its physical properties with those of ordinary water.
Ans. Heavy water can be prepared by exhaustive electrolysis of water. Comparison of physical properties of H2O and D2O.
| Property | H2O | D2O | |
| (i) | Molecular mass (g mol-1) | 18.015 | 20.027 |
| (ii) | Melting point (K) | 273.0 | 276.8 |
| (iii) | Boiling point (K) | 373.0 | 374.4 |
| (iv) | Density (298 K) g cm-3 | 1.0000 | 1.1059 |
| (v) | Enthalpy of vapourisation (kJ mol-1) | 40.66 | 41.61 |
Q.38. Write one chemical reaction for the preparation of D2O2.
Ans. D2O2 can be prepared by the reaction of D2S04 dissolved in water over BaO2.
BaO2 + D2S04 → BaS04 + D2O2
Q.39. Calculate the strength of 5 volume H2O2 solution.
Ans. 5 volume H2O2 solution means that 1 L of 5 volume H2O2 on decomposition gives 5L of O2 at NTP.
H2O2 → 2H20 + O2
68 g 22.4 L at NTP
22.4 L of O2 at NTP is produced from H2O2 = 68 g
5 L of O2 at NTP is produced from H2O2 = 68/22.4 x 5 g
But 5 L of O2 at NTP is produced from 1 L o f 5 volume H2O2
Strength of H2O2 in 5 volume H2O2 = 15.18 g/L
Percentage strength of H2O2 solution = 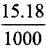 x 100 = 1.518%
x 100 = 1.518%
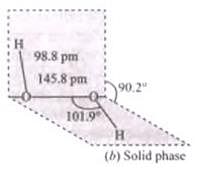
Q.40.(1) Draw the gas phase and solid phase structure of H2O2.
(2) H2O2 is a better oxidising agent than water. Explain.
Ans.(1) Structure of H2O2 is slightly different in gas phase and solid phase.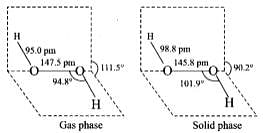
(2) H2O2 is a better oxidizing agent than water. H2O2 acts as an oxidizing agent in acid as well as in alkaline medium. E° = 1.77 V
E° = 1.77 V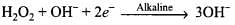 E° = 0.88 V
E° = 0.88 V
Oxidation state of oxygen changes from -1 to -2. Oxidising nature of H2O2 can be interpreted on account of possession of labile oxygen.
H2O2 → H2O + O
When water acts as an oxidizing agent, it is reduced to H2. Water reacts with number of active metals whose electrode potential is less than -0.83 V.

Q.41. Melting point, enthalpy of vapourisation and viscosity data of H2O and D2O is given below :
| H2O | D2O | |
| Melting point / K | 373.0 | 374.4 |
| Enthalpy of vapourisation at (373 K)/ kJ mol–1 | 40.66 | 41.61 |
| Viscosity/centipoise | 0.8903 | 1.107 |
On the basis of this data, explain in which of these liquids intermolecular forces are stronger?
Ans. The melting point, enthalpy of vapourisation and viscosity values of all these items depend upon the intermolecular forces of attraction. Since their values are higher for D2O as compared to those of H2O, therefore, intermolecular forces of attraction are stronger in D2O than in H2O.
Q.42. Dihydrogen reacts with dioxygen (O2) to form water. Write the name and formula of the product when the isotope of hydrogen which has one proton and one neutron in its nucleus is treated with oxygen. Will the reactivity of both the isotopes be the same towards oxygen? Justify your answer.
Ans. The isotope of hydrogen which contains one proton and one neutron is deuterium (D). Thus, when dideuterium reacts with dioxygen, deuterium oxide, i.e., heavy water is produced.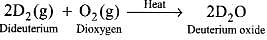
The reactivity of H2 and D2 towards oxygen will be different. Since the D – D bond is stronger than H – H bond, therefore, H2 is more reactive than D2 towards reaction with oxygen.
Q.43. Explain why HCl is a gas and HF is a liquid.
Ans. Due to greater electronegativity of F over Cl, F forms stronger H-bonds as compared to Cl. As a result, more energy is needed to break the H-bonds in HF than in HCl and hence the b.p. of HF is higher than that of HCl. Consequently, H-F is liquid while HCl is a gas at room temperature.
Q.44. When the first element of the periodic table is treated with dioxygen, it gives a compound whose solid state floats on its liquid state. This compound has an ability to act as an acid as well as a base. What products will be formed when this compound undergoes autoionisation?
Ans. The first element is hydrogen and its molecular form is dihydrogen (H2). It reacts with oxygen to form water whose solid state is ice which is lighter than water and floats over water. Water is amphoteric in nature, i.e., it acts as an acid in the presence of strong bases and acts as a base in the presence of strong acids.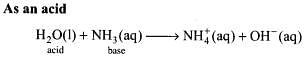
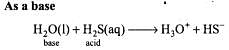
Due to amphoteric nature, water undergoes self ionization as
Q.45. Rohan heard that instructions were given to the laboratory attendent to store a particular chemical i.e., keep it in the dark room, add some urea in it, and keep it away from dust. This chemical acts as an oxidising as well as a reducing agent in both acidic and alkaline media. This chemical is important for use in the pollution control treatment of domestic and industrial effluents.
(1) Write the name of this compound.
(2) Explain why such precautions are taken for storing this chemical.
Ans.(1) The name of the compound is H2O2. It acts as an oxidizing as well as reducing agent in both acidic and basic media.
(2) H2O2 is decomposed by light and dust particles. Urea is added as a negative catalyst, i.e., to check its decomposition.
2H2O2(l)→2H2O(l) + O2(g)
Because of the oxidizing properties, H2O2 is widely used to control pollution by oxidation of harmful cyanides and obnoxious smelling sulphides present in domestic and industrial effluents. It also helps in sewage disposal by supplying O2 for oxidation of organic matter present – in sewage waters.
Q.46. Give reasons why hydrogen resembles alkali metals?
Ans. Hydrogen has electronic configuration Is1. On one hand, its electronic configuration is similar to the outer electronic configuration (ns1) of alkali metals, which belong to the first group of the periodic table Hydrogen, therefore, has resemblance to alkali metals, which lose one electron to form unipositive ions.
Q.47. Hydrogen generally forms covalent compounds. Give reason.
Ans. Because of ionization enthalpy, hydrogen resembles more with halogens. ΔH of Li is 520 kJ mol-1. F is 1680 kJ mol-1 and that of H is 1312 kJ mol-1. Like halogens, it forms a diatomic molecule, combines with elements to form hydrides and a large number of covalent compounds.
Q.48. Why is the Ionisation enthalpy of hydrogen higher than that of sodium?
Ans. In sodium the last shell electron is in 3s1 after loosing that electron, it can acquire the configuration of Ne. Whereas in hydrogen, the electron is in s-orbital.
Q.49. Basic principle of hydrogen economy is transportation and storage of energy in the form of liquid or gaseous hydrogen. Which property of hydrogen may be useful for this purpose? Support your answer with the chemical equation if required.
Ans. Hydrogen economy (Hydrogen as fuel):
(i) The electricity cannot be stored to mn automobiles It is not possible to store and transport nuclear energy Hydrogen is an alternative source of energy and hence called as ‘hydrogen economy'. Hydrogen has some advantages as fuel.
(ii) Available in abundance tn combined form as water.
(iii) On combustion produces H2O. Hence pollution free.
(iv) H2-O2 fuel cell give more power.
(v) Excellent reducing agent. Therefore, can be used as substitute of carbon in reduction for processes in industry.
Q.50. What is the importance of heavy water?
Ans. Heavy water can be used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
Q.51. Write the Lewis structure of hydrogen peroxide.
Ans.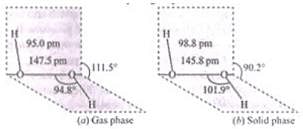
(a) H2O2 structure in gas phase, dihedral angle is 111.5°
(b) H2O2 structure in solid phase at 110 k, dihedral angle is 90.2°
Q.52. An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. Illustrate it with the help of a chemical equation.
Ans. (i) Oxidising action in acidic medium: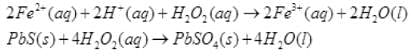
(ii) Reducing action in acidic medium: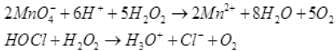
Q.53. With the help of suitable examples, explain the property of H2O2 that is responsible for its bleaching action?
Ans. H2O2 decomposes slowly on exposure to light.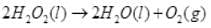
In daily life it Is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
Q.54. Why is water molecule polar?
Ans. Water molecule is polar because in the gas phase water is a bent molecule with a bond angle of 104.5°, a11d O-H bond length of 95.7 pm.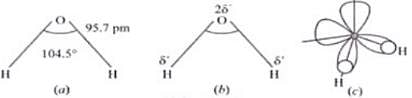
H2O molecule
(a) The bent structure of water
(b) The water molecule as a dipole and
(c) The orbital overlap picture in water molecule
Q.55. Why does water show high boiling point as compared to hydrogen sulphide?Give reasons for your answer.
Ans. Because of hydrogen bonding water has high boiling point than H2S as in H2S hydrogen bonding is absent.
Q.56. Why can dilute solutions of hydrogen peroxide not be concentrated by heating. How can a concentrated solution of hydrogen peroxide be obtained?
Ans. Hydrogen peroxide cannot be concentrated by heating as it can cause bums on heating.It can be extracted with water and concentrated to ∼30% (by mass) by distillation under reduced pressure. It can be further concentrated to ∼85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2.
Q.57. Why is hydrogen peroxide stored in wax lined bottles?
Ans. Hydrogen peroxide is stored in wax lined bottles because H2O2 decomposes slowly on exposure to light.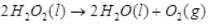
Q.58. Why does hard water not form lather with soap?
Ans. Hard water forms scum/precipitate with soap. Soap, containing sodium stearate (C17 H35COONa). reacts with hard water to precipitate out Ca/Mg stearate. It is. therefore, unsuitable for laundry. It is harmful for boilers as well, because of deposition of salts in the form of scale.
Q.59. Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Ans. Because H2SO4 can activate the decomposition of H2O2.
Q.60. How will you account for 104.5° bond angle in water?
Ans.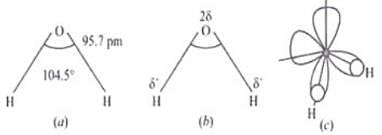
In water hybridisation of oxygen is sp3. Angle should be 109°(approx.) but due to LP - LP repulsion bond angle reduce to 104.5°.
Q.61. Write redox reaction between fluorine and water.
Ans.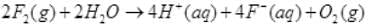
Q.62. Write two reactions to explain amphoteric nature of water.
Ans.
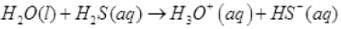
MATCHING TYPE QUESTIONS
Q.63. Correlate the items listed in Column I with those listed in Column II. Find out as many correlations as you can.
| Column I | Column II |
| (i) Synthesis gas | (a) Na2[Na4(PO3)6] |
| (ii) Dihydrogen | (b) Oxidising agent |
| (iii) Heavy water | (c) Softening of water |
| (iv) Calgon | (d) Reducing agent |
| (v) Hydrogen peroxide | (e) Stoichiometric compounds of s-block elements |
| (vi) Salt like hydrides | (f) Prolonged electrolysis of water |
| (g) Zn + NaOH | |
| (h) Zn + dil. H2SO4 | |
| (i) Synthesis of methanol | |
| (j) Mixture of CO and H2 |
Ans. (i) → (j); (ii) → (h); (iii) → (f) : (iv) → (a); (v) → (b); (vi) → (e)
Q.64. Match Column I with Column II for the given properties/applications mentioned therein.
| Column I | Column II |
| (i) H | (a) Used in the name of perhydrol. |
| (ii) H2 | (b) Can be reduced to dihydrogen by NaH. |
| (iii) H2O | (c) Can be used in hydroformylation of olefin. |
| (iv) H2O2 | (d) Can be used in cutting and welding. |
Ans. (i) → (d); (ii) → (b); (iii) → (c); (iv) → (a)
Q.65. Match the terms in Column I with the relevant item in Column II.
| Column I | Column II |
| (i) Electrolysis of water produces | (a) atomic reactor |
| (ii) Lithium aluminium hydride is used as | (b) polar molecule |
| (iii) Hydrogen chloride is a | (c) recombines on metal surface to generate high temperature |
| (iv) Heavy water is used in | (d) reducing agent |
| (v) Atomic hydrogen | (e) hydrogen and oxygen |
Ans. (i) → (e); (ii) → (d); (iii) →(b); (iv) → (a); (v) → (c)
Q.66. Match the items in Column I with the relevant item in Column II.
| Column I | Column II |
| (i) Hydrogen peroxide is used as a | (a) zeolite |
| (ii) Used in Calgon method | (b) perhydrol |
| (iii) Permanent hardness of hard water is removed by | (c) sodium hexametaphosphate |
| (d) propellant |
Ans. (i) → (b); (ii) → (c); (iii) →(a)
ASSERTION AND REASON TYPE
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the options given below each question.
Q.67. Assertion (A) : Permanent hardness of water is removed by treatment with washing soda.
Reason (R) : Washing soda reacts with soluble magnesium and calcium sulphate to form insoluble carbonates.
(1) Statements A and R both are correct and R is the correct explanation of A.
(2) A is correct but R is not correct.
(3) A and R both are correct but R is not the correct explanation of A.
(4) A and R both are false.
Ans. (1)
Solution.
Statements A and R both are correct and R is the correct explanation of A.
Q.68. Assertion (A) : Some metals like platinum and palladium, can be used as storage media for hydrogen.
Reason (R) : Platinum and palladium can absorb large volumes of hydrogen.
(1) Statements A and R both are correct and R is the correct explanation of A.
(2) A is correct but R is not correct.
(3) A and R both are correct but R is not the correct explanation of A.
(4) A and R both are false.
Ans. (i)
Solution.
Statements A and R both are correct and R is the correct explanation of A.
LONG ANSWER TYPE QUESTIONS
Q.69. Atomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
Ans. Atomic hydrogen is very reactive whereas molecular hydrogen are quite stable. The chemical behaviour of dihydrogen (and for that matter any molecule) is determined, to a large extent, by bond dissociation enthalpy. The H-H bond dissociation enthalpy is the highest for a single bond between two atoms of any element. As a result, molecular hydrogen reacts only with a few elements.
Q.70. How can D2O be prepared from water? Mention the physical properties in which D2O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium.
Ans. D20 can be prepared by prolonged electrolysis of water. Due to high molecular mass D20 differ from water.
NaOH + D2O → NaOD + HOD
HCl+D2O → DCl + HOD
NH4Cl + D2O → NH3DCI + HOD
Physical Properties of H2O and D2O
| Property | H2O | D2O |
| Molecular mass (g mol-1) | 18.0151 | 20.0276 |
| Melting point/K | 273.0 | 276.8 |
| Boiling point/K | 373.0 | 374.4 |
| Enthalpy of formation/kJ mol-1 | -285.9 | -294.6 |
| Enthalpy of Vaporisation (373 K)/kJmol-1 | 40.66 | 41.61 |
| Enthalpy of fusion/kj mol-1 | 6.01 | - |
| Temp of max.density/K | 276.98 | 284.2 |
| Density (298K)/g cm-3 | 1.0000 | 1.1059 |
| Viscosity/centipoise | 0.8903 | 1.107 |
| Dielectric constant/C2/N.m2 | 78.39 | 78.06 |
| Electrical conductivity (293 K /ohm-1 cm-1) | 5.7 x 10-8 | - |
Q.71. How will you concentrate H2O2? Show differences between structures of H2O2 and H2O by drawing their spatial structures. Also mention three important uses of H2O2.
Ans. Acidifying barium peroxide and removing excess water by evaporation under reduced pressure gives hydrogen peroxide. It is extracted with water and concentrated to ∼30% (by mass) by distillation under reduced pressure. It can be further concentrated to ∼85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2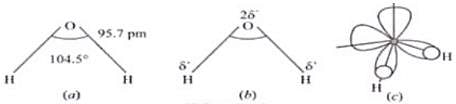
H2O molecule
(a) The bent structure of water
(b) The water molecule as a dipole and
(c) The orbital overlap picture in water molecule
(i) As an antiseptic it is solid in market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per- carbonate. It is employed in the industries as a bleaching agent for textiles.
Q.72. (1) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
(2) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations.
Ans. (1) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols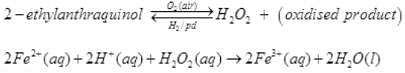
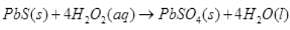
(2) (a) Reducing action in acidic medium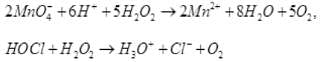
(b)Oxidising action in basic medium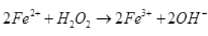
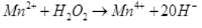
(c) 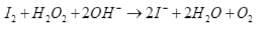
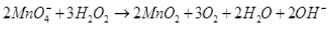
Q.73. What mass of hydrogen peroxide will be present in 2 litres of a 5 molar solution? Calculate the mass of oxygen which will be liberated by the decomposition of 200 mL of this solution.
Ans. Mass of H2O2 = 68 g.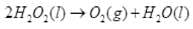
Mass of oxygen produced= 3.2 g
Q.74. A colourless liquid ‘A’ contains H and O elements only. It decomposes slowly on exposure to light. It is stabilised by mixing urea to store in the presence of light.
(i) Suggest possible structure of A.
(ii) Write chemical equations for its decomposition reaction in light.
Ans. (i) A is H2O2. Structure of H2O2 is given below.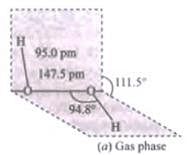
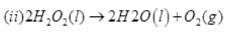
Q.75. An ionic hydride of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al2Cl6.
Ans. Lithium hydride is rather unreactive at moderate temperatures with O2 or Cl2.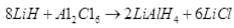
Q.76. Sodium forms a crystalline ionic solid with dihydrogen. The solid is nonvolatile and non- conducting in nature. It reacts violently with water to produce dihydrogen gas. Write the formula of this compound and its reaction with water. What will happen on electrolysis of the melt of this solid.
Ans. Saline hydrides react violently with water producing dihydrogen gas.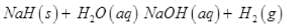
Their melts conduct electricity and on electrolysis liberate dihydrogen gas at anode, which confirms the existence of H ion.
|
130 videos|235 docs|178 tests
|
FAQs on NCERT Exemplar: Hydrogen (Old NCERT) - Chemistry for Grade 11
| 1. What is the chemical formula of hydrogen? |  |
| 2. How is hydrogen used as a fuel? |  |
| 3. What are the advantages of using hydrogen as an alternative fuel? |  |
| 4. How is hydrogen produced on a large scale? |  |
| 5. What are the potential applications of hydrogen in the future? |  |
|
Explore Courses for Grade 11 exam
|

|

















