Nitrenes and Carbenes: Stability & Reactions | Organic Chemistry PDF Download
A. What are Nitrenes?
Nitrenes are nitrogen analogues of carbenes. The nitrogen atom possesses only six valence electrons; in nitrenes the triplet is lower in energy than the singlet state. (i) The nitrogen analouges of carbenes are called nitrenes.
(ii) There is possibility of two spin states for nitrenes depending on whether the two-non-bonding electrons (the normal nitrogen lone pair remains paired) have their spins paired or parallel.
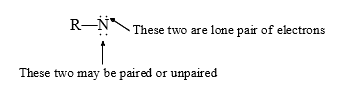
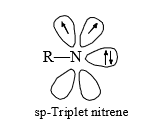
In general nitrenes obey Hunds rule and are ground state triplet with degenerate sp-orbitals containing a single electron each.
(B) Generation of nitrenes
1. From 1, 1-Elimination
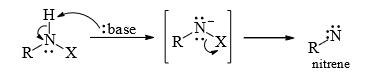
2. From azides
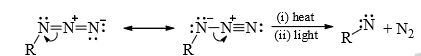
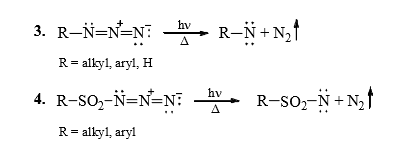
C. Fate of Nitrenes
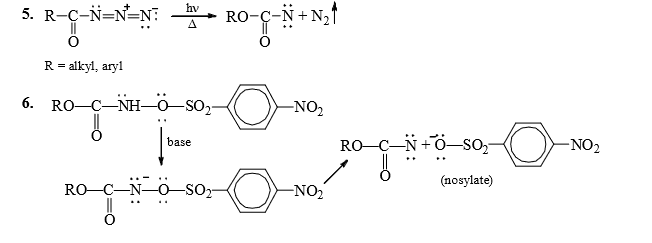
1. Hoffmann Rearrangement
Mechanism
2. Curtius Rearrangement
Mechanism
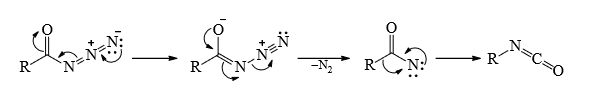
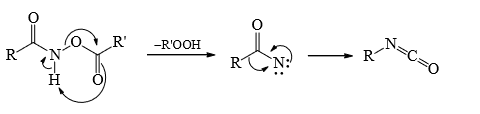
3. Lossen Rearrangement
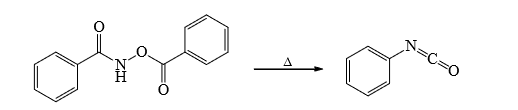
Mechanism
4. Beckmann Rearrangement
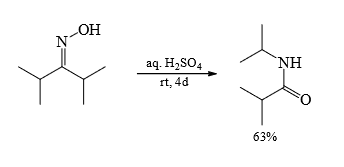
Mechanism
5. Schmidt Rearrangement
Schmidt Rearrangement of Ketones
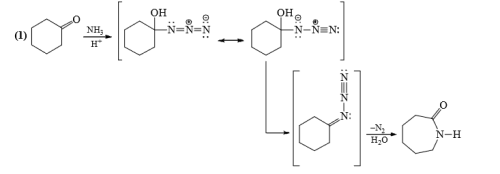
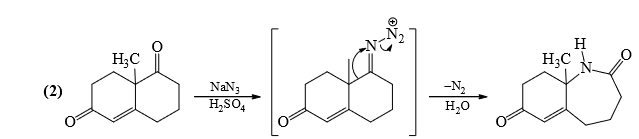
6. Schmidt Reaction
CARBENES
A. What are Carbene?
(i) Carbenes are neutral, divalent, highly reactive carbon intermediates represented by
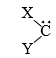
(ii) Carbenes are related to carbanions through the α-elimination reaction (equation 1)
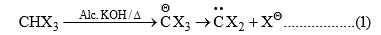
(iii) Carbenes can also be considered as conjugate base of carbocations. (equation 2)
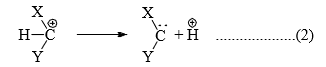
(iv) The simplest carbene is CH2 which is known as methylene. Substituted carbenes are simply narned as substituted derivative of carbenes;
For example:
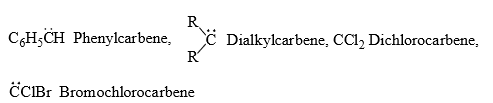
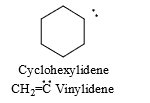
(v) The exceptions to this are carbenes in which divalent carbon is part of the ring or is doubly bonded. These are named using the-ylidene suffix.
For example:
(vi) Carbenes are usually throught to be sp2 hybridised. There are two bonding electrons (both in sp2-orbitals) and two non-bonding electron associated with the carbon. Since, the non bonding electrons can have their spins paired or paralled, there is the possibility of two electronic arrangement or spin states. The singlet carbene (spin multiplicity S = 1) has the spins of its non-bonding electrons paired. This non-bonding electrons pair is in an sp2orbital leaving a vacant p-orbital.
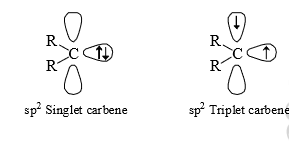
However, in triplet carbene the non-bonding electrons have paralled spins and both the sp2 and p-orbitals contain one electron each (triple carbene)
Hence triplet carbenes (multiplicity S = 3) with their unpaired electrons exhibit propoerties of diradicals, and under the right conditions, can be detected by ESR spectroscopy.
If carbenes were linear, the carbene carbon will be sp-hybridised. Two p-orbitals will remain unhybridised, and the two non-bonding electrons should go one into each with spin parallel according to Hund’s rule because these porbitals would be degenerate. This carbene will be sp-triplet.
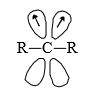
An sp2 hybridised carbene would have three (lower energy) sp2 orbitals and one (high energy) p-orbital in which to distribute its six electrons. There are two ways of doing this. Either all of the six electrons can be paired, with each pair occupying one of the sp2 orbitals or two of the electrons can remain unpaired. With one electron in each of the p-orbitals and one of the sp2 orbtials.
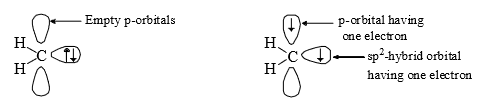
Triplet carbenes have two unpaired electrons, one in each of an sp2 and a p-orbital, while singlet carbene have a pair of electrons in a non-bonding sp2 orbital and have an empty p-orbital. The orbital occupancy explains the smaller bond angle in singlet carbenes, which have an electronrepelling lone pair in an sp2 orbital.
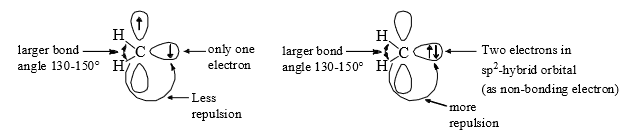
In most of the cases triplet carbon be is more stable than the singlet carbene because the energy to be gained by bringing the electron in the p-orbital down into the sp2 orbital is insufficient to overcome the repulsion that exists between two electrons in a single orbital.
(B) Generation of carbenes: Carbenes are usually formed from precursons by the loss of small stable molecules.
(1) From diazo compounds: Diazo compounds easily decompose thermally and photochemically to give carbenes.
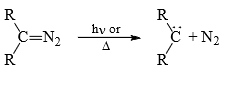
The most common diazo comopunds is diazomethane (CH2N2). But this method is not a very practical method of generating carbenes because of the explosive nature of diazoalkanes. However, diazocarbonyl compounds are stable, non-exaplosive and are frequently used for the preparation of octyl carbenes.
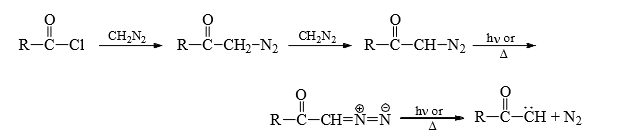
Now-a-days transition metals such as copper or rhodium are used for the conversion of diazoacyl compounds into carbene.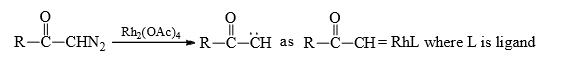
In fact true carbenes are not formed in thise case. Carbene are obtained as metal carbene complex known as carbenoids.
Rhodium and copper carbenoids are unstable but tungsten and chromium form stable carbenoids known as metallocarbenes are Fisher carbenes.
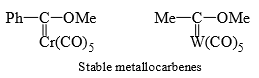
(2) From Tosylhydrazones: Carbonyl compounds react with tosylhydrazine in the presence of alkoxide to give tosylhydrazone which on strong heating gives carbenes.
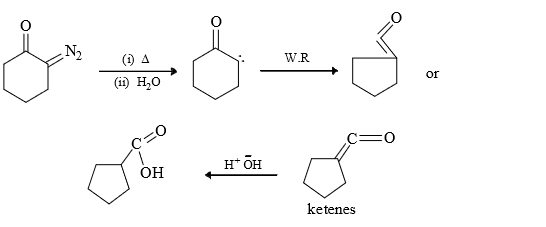
3) From ketones: Thermal or photochemical decomposition of ketenes form carbenes.
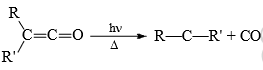
The reaction is not widely used since ketenes are not readily available precursors and tend to polymerise under the reaction conditions.
(4) From ylides: Carbenes may be obtained from phosphorus, sulphur or nitrogen ylides on heating or photochemically.
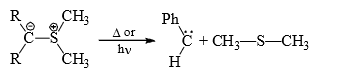
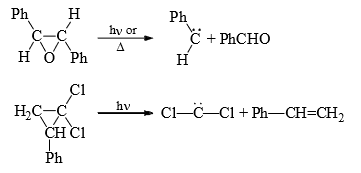
(5) From small ring (three membered) compounds: Three membered rings, which have a high ground state energy because of the angle strain, will often decomposes to give carbenes to give carbenes on heating or irradiation.
(6) From Gem dihalides and trihalides: Gem dihalides and gem trihalides having at least one a-hydrogen give carbene by α-elimination reaction in the presence of strong base.
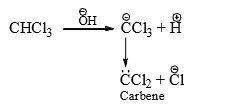
The ease of elimination depends on the leaving power of halo group and the order is as follows: I > Br > Cl > F. Thus, CHF2Cl gives exclusively difluorocarbene.
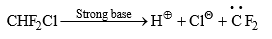
The reactions are carried out in the presence of organic solvent using a strong base mainly sodium or potassium salt of tertiary butyl alcohol. But nowadays the use of phase transfer catalyst is more commonly used. The method involves taking a two-phose mixture of chloroform and 50% aqueous sodium hydroxide solution and adding a PhCH2N(C2H5)3Cl which acts as the phase transfer catalyst.
α-elimination always gives singlet carbenes.
(7) From acid chloride (Diazoketone):
Note : This reaction is carried in presence of HCl to remove 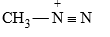
Above preparation of carbene is used in Arndt-Eistert synthesis.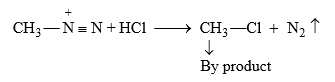
(8) 1, 2, 3 tri carbonyl compounds :
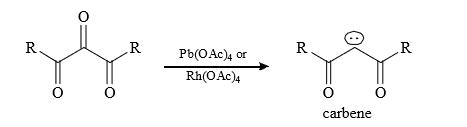
(9) From degradation of some special compounds
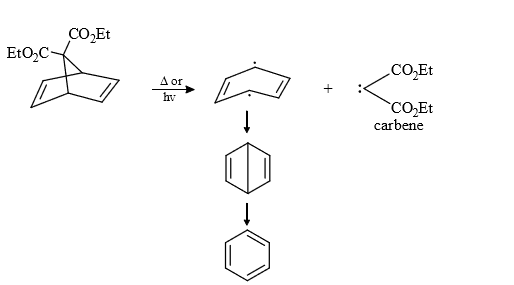
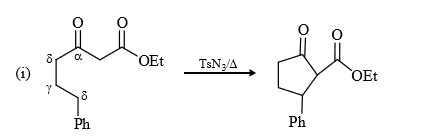
(10) From active methylene compounds : Active methylene group can be converted into carbene by reaction with diazotransfering compounds.
e.g., Tosyl (Ts N3) azide
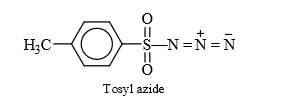
(11) From Diazirine : 3 membered unsaturated ring
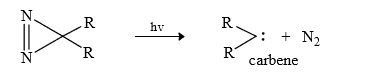
(12) From Hg-compounds : (R—Hg—CX3) group
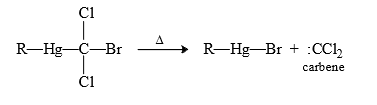
(C) Fate of Carbenes:
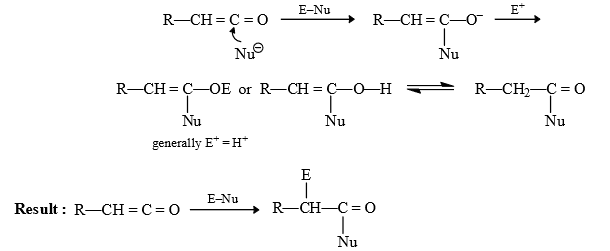
(1) Arndt Eistert synthesis : By this reacton next homologue of carboxylic acid is prepared.
Reaction of Ketene:
(2) Wolf Rearrangement: It involves alkyl groups shift with retention of configuration.
(3) Gem di addition:
Note : Carbene also show gem di addition
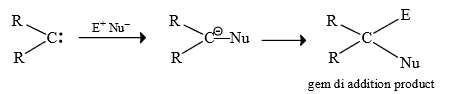
Carbene show W.R. as well as gem di addition this will depends upon availability of reagent. If the reagent (E.Nu) is present insitu than gem diaddition takes place and if it is added later on than W.R. take place firstly.
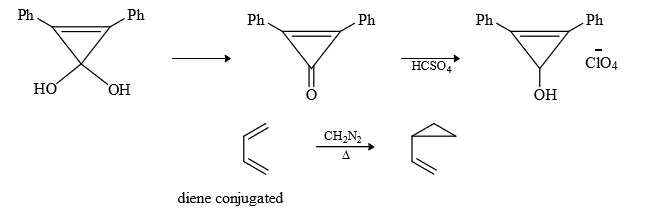
Result : Singlet adds to more substituted double bond while triplet adds to less substituted double bond (C = C)
(4) Insertion in benzene ring :
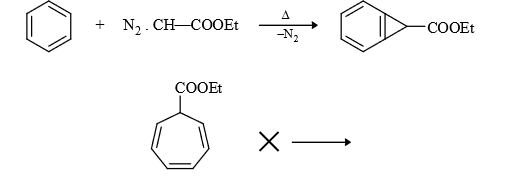
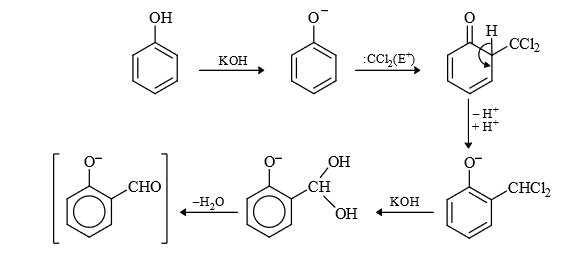
(5) Reimer Teimann Reaction :
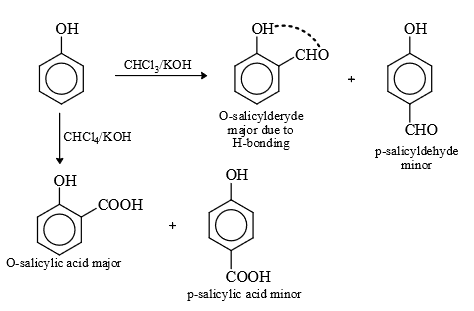
Mechanism :
(6) Insertion of δ position
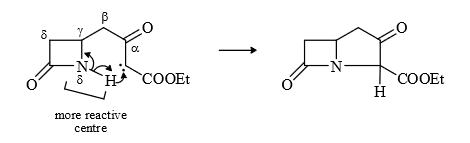
If δ – H is absent then β position react
(7) Sapiro reaction or Shapiro reaction : It is used in the preparation of alkene by the use of carbene. In this reaction firstly aldehyde ketone groups is converted in to Ts hydrazone by reaction with Ts hydrazine, then this dydrazone is treated with base. The product of reaction depend upon nature of base whether it is weak or strong.
With weak base :
(ii) With strong base : Usually in this condition 2 equivalent of strong base are used, one will involve in the formation of carbene and other is involved in the release of acidic H and help in the formation of vinyl anion.
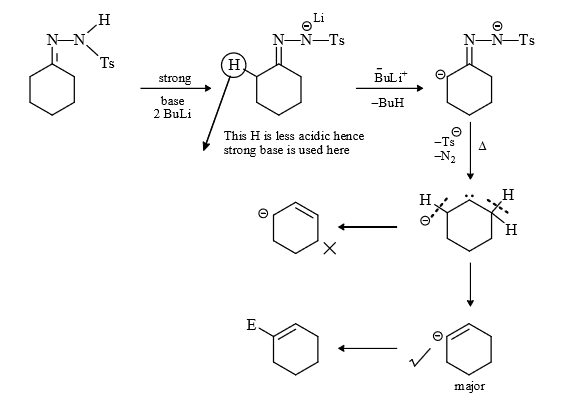

Note : In Shapiro reaction the nature of alkene formed depend upon basic strength if weak base is used then more stable i.e., more substituted alkene is formed but if strong base is used then less substituted alkene is formed i.e., with weak base saytzeff alkene is formed, and with strong base Hoffmann alkene is formed
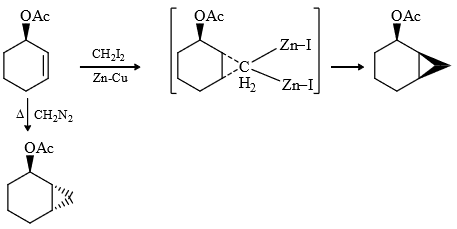
(8) Simmons-Smith Reaction
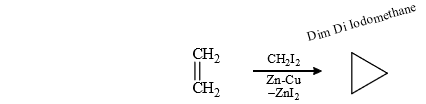
It is cyclo propanation i.e., cyclo propane ring is formed.
Mechanism :
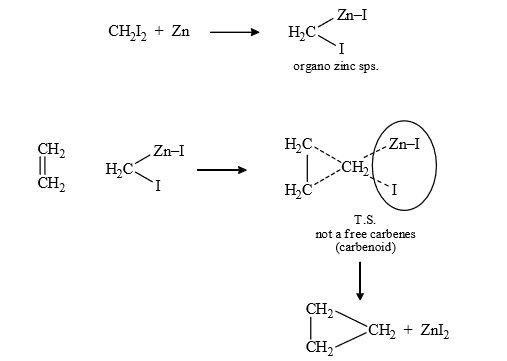
Result : (1) In this reaction pure carbene is not formed it is called carbenoid.
(2) In this addition, stereochemistry of reactant is maintained in product i.e., it is stereospecific reaction.
i.e., cis alkene give cis product and trans give trans product
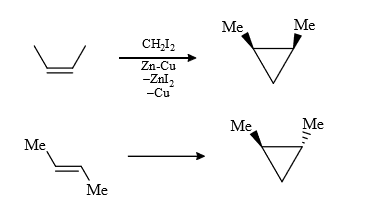
(3) It is a regio selective reaction and the carbenoid is electrophillic (less electron dense) therefore more electron dense = prefer this attack.
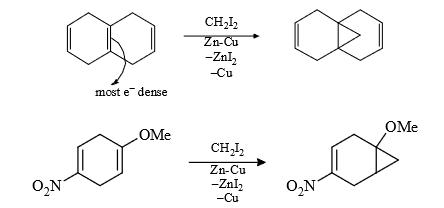
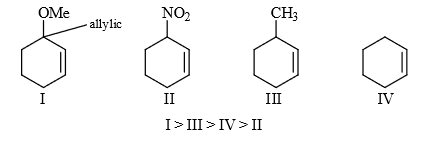
Rate of S.S. reaction
Most reactive because it’s lone pair stabilize the carpenoid.
(4) In carbenoids there is electrophilicity (e– deficiency) therefore any group (containing lone pair or π bond) will stabilized the carbenoid, therefore rate of reaction increases and stereochemistry of cyclo propane ring same that of stabhilising group, this is due to insitu formation of carbebne i.e., carbenoid.
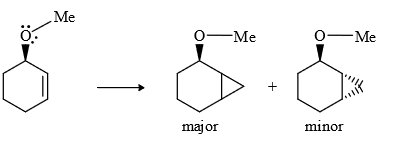
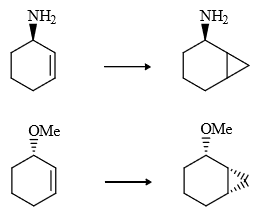
Note : The above explanation was fond to be wrong infact the driving force for same stereochemistry is the chelation of donor group Zn.
(D) Stability of carbenes: Triplet (CH2) is 32-42 kJ/more stable than singlet carbene (CH2). The triplet carbene has a lower energy because with two electrons in different orbitals there is less electrostatic repulsion than both are in same orbital. However, the nature of the substituents has a important effect onthe stability of carbenes. For example, as the carbene substituents (i.e., substituent present on divalent carbon) become better pi-electron donors, then the increased stability of singlet state and singlet state carbenes become more stable than the triplet state carbenes. Thus is a singlet carbene. A p-atomic orbital of X with a lone pair of electrons can overlap laterally with the empty p-atomic orbital of the singlet carbene thereby stabilising the singlet state. This stabilisation is not possible in triplet state whose p-atomic orbitals are not empty.
The resonance structures are:
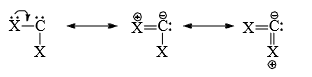
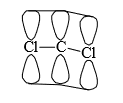
Among dihalocarbenes, the most stable is the difluorocarbene. Stability of difluorocarbene can be explained as follows: Since, fluorine and carbon are in the same period, their p-atomic orbitals about the same size permitting more efficiency overlapping. “Furthermore, of the C—X bonds, the C—F bond length is the shortest which again provides for more extensive lateral overlap of the respective p-atomic orbitals. On this basis it can be concluded that singlet CF2 is more stable than CCl2 which is more stable than CBr2. There can be other reasons for the extra stability of singlet state. Cycloheptatirenylidene is such a carbene.
|
44 videos|102 docs|52 tests
|
FAQs on Nitrenes and Carbenes: Stability & Reactions - Organic Chemistry
| 1. What are nitrenes and carbenes? |  |
| 2. How are nitrenes and carbenes stabilized? |  |
| 3. What are the main reactions of nitrenes and carbenes? |  |
| 4. How do nitrenes and carbenes participate in cycloaddition reactions? |  |
| 5. What are the applications of nitrenes and carbenes in organic chemistry? |  |

















