Organic Reactions With Mechanism and Applications (Part -3) | Organic Chemistry PDF Download
⇒ Reimer - Tiemann reaction:
Formylation of phenols with chloroform in alkaline solution is known as Reimer-Tiemann reaction (RTR). The method is applicable to phenols and some heterocyclic compounds.
A mixture of ortho - and para - isomers is obtained in which the ortho isomer predominates. If one of the ortho positions is occupied, the para-isomer is the major product.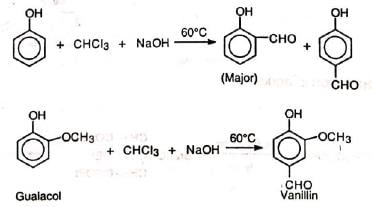 The reaction is carried out by refluxing an alkaline solution of phenol and chloroform at 60°C for sometime (1/2 h). Excess chloroform is distilled off, the mixture acidified with sulphuric acid and steam distilled. Unreacted phenol and the ortho-isomer distil over leaving behind the para-isomer. The two isomers are further purified by sodium bisulphite. The yield is low.
The reaction is carried out by refluxing an alkaline solution of phenol and chloroform at 60°C for sometime (1/2 h). Excess chloroform is distilled off, the mixture acidified with sulphuric acid and steam distilled. Unreacted phenol and the ortho-isomer distil over leaving behind the para-isomer. The two isomers are further purified by sodium bisulphite. The yield is low.
Mechanism:
It is believed that chloroform and sodium hydroxide react to produce an electron-deficient electrophile, dichlorocarbene (:CCI2).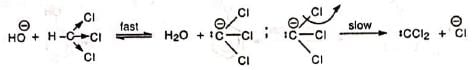 Dichlorocarbene then attacks the aromatic ring to yield a dichloro anion (i) which takes up a proton from the solvent. The product (ii) tautomerizes to o-dichloromethyl phenol which after hydrolysis and acidification gives salicylaldehyde.
Dichlorocarbene then attacks the aromatic ring to yield a dichloro anion (i) which takes up a proton from the solvent. The product (ii) tautomerizes to o-dichloromethyl phenol which after hydrolysis and acidification gives salicylaldehyde.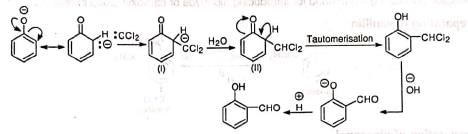 That the reacting species is dichlorocarbene is supported by the following:
That the reacting species is dichlorocarbene is supported by the following:
(i) Dichlorocarbene is known to cause ring expansion which is observed in the reaction of pyrrole with chloroform and alkali.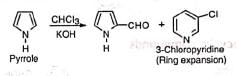 (ii) When p-cresol instead of phenol is used besides the normal aldehyde, a ketone (III) with dichloromethyl group is obtained as a by-product.
(ii) When p-cresol instead of phenol is used besides the normal aldehyde, a ketone (III) with dichloromethyl group is obtained as a by-product.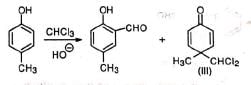 The compound (III) may be explained to be formed by the attack of some :CCI2 at the para position and subsequent gain of a proton from the solvent. As this para position has no ring H that can be lost as proton to re-aromatise the ring, the reaction does not proceed further.
The compound (III) may be explained to be formed by the attack of some :CCI2 at the para position and subsequent gain of a proton from the solvent. As this para position has no ring H that can be lost as proton to re-aromatise the ring, the reaction does not proceed further.
Due to its insolubility in basic aqueous medium and crowding around chlorine atoms, the dichloro compound (III) is not hydrolysed.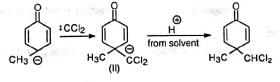 The presence of negative groups such as CN, COOH, NO2, SO3H, etc., decreases the yield.
The presence of negative groups such as CN, COOH, NO2, SO3H, etc., decreases the yield.
The yield is nearly 50% and a large amount of phenol remains unreacted. This is probably due to the formation of diphenylacetal derivative.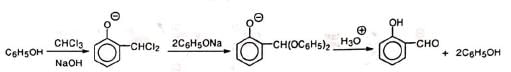 Carbon tetrachloride in place of chloroform under RTR condition gives salicylic acid.
Carbon tetrachloride in place of chloroform under RTR condition gives salicylic acid.
Applications:
The reaction affords a good method for introducing aldehyde or carboxyl group in phenols.
(i) Preparation of vanillin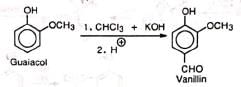 (ii) Preparation of piperonal
(ii) Preparation of piperonal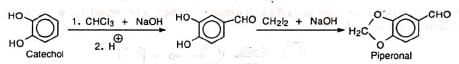 (iii) Formylation of heterocyclic compounds
(iii) Formylation of heterocyclic compounds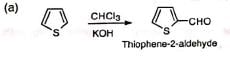
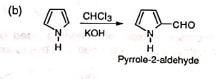
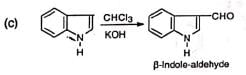
The compound has been used to synthesize the amino acid, tryptophan.
(iv) Formylation of naphthol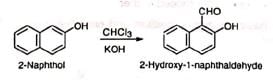 (v) Preparation of acid
(v) Preparation of acid
When carbon tetrachloride in place of chloroform is used, a carboxyl group is introduced.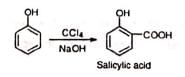 (vi) Abnormal Reimer-Tiemann reaction
(vi) Abnormal Reimer-Tiemann reaction
1. Pyrrole undergoes normal and abnormal RTR
Normal reaction—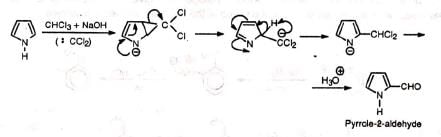 Abnormal reaction (Ring expansion)—
Abnormal reaction (Ring expansion)—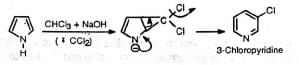 The reaction has been used to prepare pyridine and its derivatives.
The reaction has been used to prepare pyridine and its derivatives.
(vii) Certain phenolic compounds also exhibit normal and abnormal RTR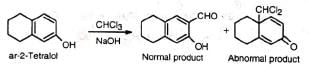 The abnormal product may be reduced and then oxidized to give a product with angular methyl group. The process has been utilized in steroid chemistry.
The abnormal product may be reduced and then oxidized to give a product with angular methyl group. The process has been utilized in steroid chemistry.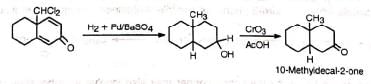
⇒ McMurry coupling:
The McMurry coupling is the reaction of two carbonyl functional groups to establish a new double bond between the carbons of the carbonyl groups. The reaction is mediated by low-valent titanium reagents, which may be generated through the combination of titanium chlorides with any of a number of reducing agents. The McMurry coupling is useful for the construction of sterically hindered alkenes, but has limited scope due to a lack of stereochemical control and statistical mixtures of products in mixed-coupling reactions.
Introduction:
The formation of alkenes as minor products in pinacol couplings of aromatic carbonyl compounds with aluminum amalgam was first reported in 1970. Since then, the reductive coupling of carbonyl compounds to afford alkenes has been developed into a useful synthetic method, most notably by McMurry and colleagues. The modern McMurry coupling employs low-valent titanium generated from a titanium source and a reducing agent (Eq. 1), and the scope of the reaction has benefited from the development of several titanium-reductant combinations. Aldehydes and ketones may be coupled in an intra- or intermolecular fashion to afford alkenes that may be difficult to access using other methods. Carboxylic acid derivatives such as esters, amides, and thioesters are amenable to coupling in some cases. In general, the (E)-isomer of product is favored over the (Z)-isomer, although mixtures may result when the substituents of the carbonyl groups are similarly sized. One-and two-electron transfer mechanisms have been postulated for the McMurry coupling and the details of its mechanism remain unknown. When the steric environments and reduction potentials of the carbonyl groups involved are similar, achievining selective mixed coupling (rather than a statistical mixture of homocoupling and mixed-coupling products) is often difficult. Methods that circumvent this issue have relied on the use of carbonyl equivalents such as thioacetals and geminal dihalides.
In general, the (E)-isomer of product is favored over the (Z)-isomer, although mixtures may result when the substituents of the carbonyl groups are similarly sized. One-and two-electron transfer mechanisms have been postulated for the McMurry coupling and the details of its mechanism remain unknown. When the steric environments and reduction potentials of the carbonyl groups involved are similar, achievining selective mixed coupling (rather than a statistical mixture of homocoupling and mixed-coupling products) is often difficult. Methods that circumvent this issue have relied on the use of carbonyl equivalents such as thioacetals and geminal dihalides.
Mechanism and Stereochemistry:
Prevailing Mechanism
The mechanism of the McMurry coupling is unclear at present, and isolated observations have pointed to both one-electron and two-electron mechanisms. In either case, reduction of the carbonyl group yielding an organometallic intermediate is a key first step of the process, and deoxygenation likely follows. One-electron reduction may afford two titanium ketyl radicals 1, which could subsequently couple with one another to yield titanium pinacolate 2. Elimination of two titanium oxo molecules would then occure to afford the product olefin. This mechanism is likely for aliphatic carbonyl compounds, and has been supported by electron paramagnetic resonance spectroscopy. More readily reducible aromatic carbonyl compounds undergo two-electron reduction to produce titanium ketyl anion 3, which leads to the same pinacolate 2 after addition to a second molecule of the carbonyl compound (Eq. 2).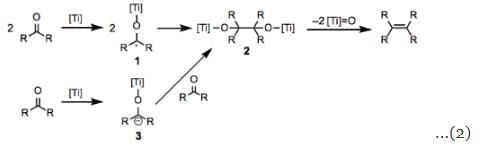 A mechanism reminiscent of olefin metathesis involving titanium carbene complexes has also been identified for hindered ketones (Eq. 3). Pinacolate 2 is not an intermediate in these reactions—the corresponding titanium pinacolates generated through other means decompose to the starting ketones rather than alkenes. Quenching the carbene intermediate with water affords the corresponding alkane.
A mechanism reminiscent of olefin metathesis involving titanium carbene complexes has also been identified for hindered ketones (Eq. 3). Pinacolate 2 is not an intermediate in these reactions—the corresponding titanium pinacolates generated through other means decompose to the starting ketones rather than alkenes. Quenching the carbene intermediate with water affords the corresponding alkane.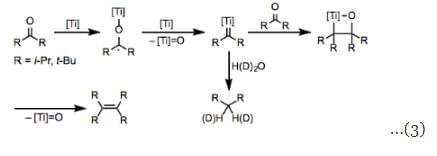 Stereochemistry
Stereochemistry
McMurry couplings generally produce mixtures of (E)- and (Z)-isomers, with the (E)-isomer predominating. Increasing the size difference between the substituents increases the selectivity for the less sterically hindered (E)-isomer.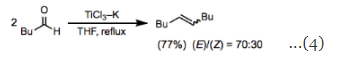 Coupling reactions between monoaryl ketones are an interesting and important exception to this rule. The tendency of these reactions to yield the (Z)-isomer as the major product when R is small has been attributed to coordination of the aryl groups to the titanium center (Eq. 5).
Coupling reactions between monoaryl ketones are an interesting and important exception to this rule. The tendency of these reactions to yield the (Z)-isomer as the major product when R is small has been attributed to coordination of the aryl groups to the titanium center (Eq. 5).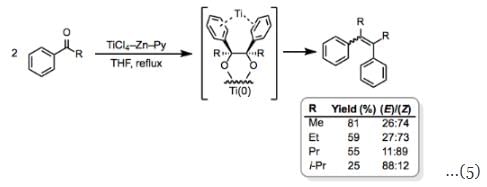
Scope and Limitations:
The McMurry coupling employing titanium requires a low-valent species, which is typically generated ‘in situ’ via treatment of a titanium halide with a reducing agent. A variety of titanium-reductant combinations have been employed for the reaction, and each has a unique scope. Reductants include zinc metal, Zn/Cu, LiAlH4, alkali and alkali earth metals, lithium arenes, and butyllithium. TiCl4 and TiCl3 are the most common titanium sources employed. In general, aliphatic ketones are more difficult to couple than aromatic ketones. For example, aliphatic ketones exclusively form pinacols in the presence of the low-valent titanium reagent generated from TiCl4 and Zn, and are poor substrates for titanium powder.
Other metals employed in McMurry couplings include zirconium, vanadium, niobium, molybdenum, tungsten, aluminum, and zinc. The practical utility of some of these metals is limited by their cost and availability, but the scope of the reaction certainly benefits from the large number of metallic reagents that may be used.
In some cases, additives can have a beneficial effect on McMurry couplings suffering from reduced yields due to pinacol formation and rearrangement. For example, amines suppress the formation of pinacols and rearrangement in the homocoupling of β-ionone (Eq. 6). Substoichiometric amounts of iodine facilitate the coupling of aliphatic carbonyl compounds by TiCl3–Li at low temperatures and short reaction times.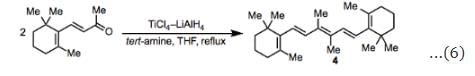
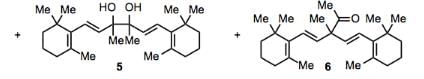
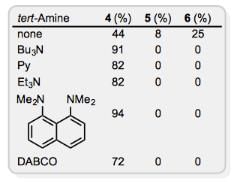 The scope of the carbonyl substrate is limited by the propensity of low-valent titanium reagents to reduce a substantial number of organic functional groups. For instance, the TiCl3–LiAlH4 system converts epoxides and bromohydrins to alkenes and mediates the deoxygenation of allylic and benzylic alcohols. Carboxylic acid substrates are generally not effective in the McMurry coupling, but intramolecular mixed-couplings of esters and amides are useful for the preparation of heterocyclic compounds (see below).
The scope of the carbonyl substrate is limited by the propensity of low-valent titanium reagents to reduce a substantial number of organic functional groups. For instance, the TiCl3–LiAlH4 system converts epoxides and bromohydrins to alkenes and mediates the deoxygenation of allylic and benzylic alcohols. Carboxylic acid substrates are generally not effective in the McMurry coupling, but intramolecular mixed-couplings of esters and amides are useful for the preparation of heterocyclic compounds (see below).
Unsaturated carbonyl compounds react smoothly in the McMurry coupling with retention of configuration at the carbon-carbon double bond. Although intermolecular coupling between two esters has not yet been reported, mixed-coupling of esters with other carbonyl functionality is known. In reactions of ketones with esters, homocoupling of the ketone can be a significant problem (Eq. 7).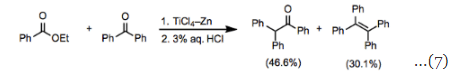 Amides are relatively versatile substrates, and both inter- and intramolecular homocoupling reactions of amides have been reported. The intramolecular coupling of amides with ketones has been employed for the synthesis of indole derivatives (Eq. 8).
Amides are relatively versatile substrates, and both inter- and intramolecular homocoupling reactions of amides have been reported. The intramolecular coupling of amides with ketones has been employed for the synthesis of indole derivatives (Eq. 8).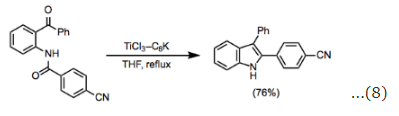 Homocoupling reactions are the most straightforward transformations that can be accomplished under the conditions of the McMurry coupling. Aliphatic and aromatic ketones can be converted into the corresponding symmetric alkenes in high yield and stereoselectivity. In reactions that could form diastereomers, selectivity for the (E)-isomer is typical (Eq. 9).
Homocoupling reactions are the most straightforward transformations that can be accomplished under the conditions of the McMurry coupling. Aliphatic and aromatic ketones can be converted into the corresponding symmetric alkenes in high yield and stereoselectivity. In reactions that could form diastereomers, selectivity for the (E)-isomer is typical (Eq. 9).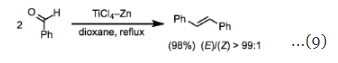 Mixed-coupling reactions between carbonyl substrates with different substitution patterns often afford a statistical mixture of products unless an excess of one of the coupling partners is employed (Eq. 10). The success of a mixed-coupling also depends on the structures of the substrates; in some cases, an excess of one of the coupling partners does not minimize homocoupling.
Mixed-coupling reactions between carbonyl substrates with different substitution patterns often afford a statistical mixture of products unless an excess of one of the coupling partners is employed (Eq. 10). The success of a mixed-coupling also depends on the structures of the substrates; in some cases, an excess of one of the coupling partners does not minimize homocoupling.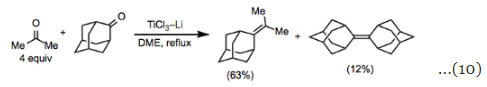 When the reduction potentials of the two substrates employed are sufficiently different, selective mixed-couplings can be accomplished using equimolar amounts of the two coupling partners. For example, monoaryl and diaryl ketones readily couple with one another in high yield in the presence of TiCl3–Zn (Eq. 11).
When the reduction potentials of the two substrates employed are sufficiently different, selective mixed-couplings can be accomplished using equimolar amounts of the two coupling partners. For example, monoaryl and diaryl ketones readily couple with one another in high yield in the presence of TiCl3–Zn (Eq. 11).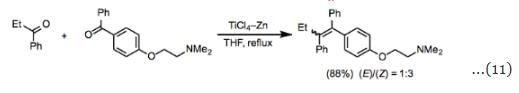 The McMurry coupling is severely limited by the drawback that mixed coupling between ketones and aldehydes is difficult to achieve. Reactions employing carbonyl equivalents such as gem-dihalides and thioacetals are amenable to mixed coupling and nicely complement the traditional McMurry coupling. Essentially, these reactions are an extension of the strategy of using coupling partners with very different reduction potentials. Esters, amides, and thioesters are useful substrates and afford electron-rich olefins (Eq. 12).
The McMurry coupling is severely limited by the drawback that mixed coupling between ketones and aldehydes is difficult to achieve. Reactions employing carbonyl equivalents such as gem-dihalides and thioacetals are amenable to mixed coupling and nicely complement the traditional McMurry coupling. Essentially, these reactions are an extension of the strategy of using coupling partners with very different reduction potentials. Esters, amides, and thioesters are useful substrates and afford electron-rich olefins (Eq. 12).
Synthetic Applications:
Generally, the McMurry coupling has been used less for synthesis than comparable olefination methods—the ubiquity of the carbonyl group in late-stage synthetic intermediates and the reductive conditions of the reaction limit its applicability to synthesis. It has been most useful for the synthesis of rather exotic compounds in which the alkene is a key disconnection. For example, the reaction is useful for the olefination of ketones containing small, strained rings (Eq. 13).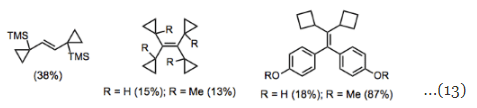 Unlike olefination methods that require the formation of a closed, four-membered intermediate such as the Wittig and Horner-Wadsworth-Emmons reactions, the McMurry coupling proceeds through open transition states and intermediates. Hence, the reaction can be used to establish severely hindered alkenes that are inaccessible by other means. Highly strained alkenes are interesting both in an academic sense and as high-energy fuels.
Unlike olefination methods that require the formation of a closed, four-membered intermediate such as the Wittig and Horner-Wadsworth-Emmons reactions, the McMurry coupling proceeds through open transition states and intermediates. Hence, the reaction can be used to establish severely hindered alkenes that are inaccessible by other means. Highly strained alkenes are interesting both in an academic sense and as high-energy fuels.
|
35 videos|92 docs|46 tests
|
FAQs on Organic Reactions With Mechanism and Applications (Part -3) - Organic Chemistry
| 1. What are organic reactions? |  |
| 2. What is a reaction mechanism? |  |
| 3. What are some examples of organic reactions with mechanisms? |  |
| 4. What are the applications of organic reactions? |  |
| 5. How can understanding organic reactions with mechanisms benefit chemists? |  |

|
Explore Courses for Chemistry exam
|

|

















