Overview: Carbohydrates | Biology and Biochemistry for MCAT PDF Download
| Table of contents |

|
| Introduction |

|
| Useful Reactions |

|
| Sugar Structures |

|
| High-Yield Terms |

|
| Passage-Based Questions and Answers |

|
Introduction
Although modern humans have been around for at least 100,000 years, one topic that seems to puzzle us to this day is our diet. In grocery stores all across the United States, you’ll find plenty of food products marketed as “low-carb” or “keto-friendly.” You may have even heard of famous athletes, such as Lebron James and Tim Tebow, sharing their results on a low-carb diet.
Despite the bad press, carbohydrates certainly played—and continue to play—a significant role in our evolutionary history and shaped who we are today. Thus, it’s no surprise that you are expected to understand them in detail as you pursue a career in medicine. In this article, we’ll discuss the basic biochemistry behind carbohydrates so that you can have a strong foundation to build on when reviewing their metabolism.
Useful Reactions
(a) Fischer and Haworth projections
- Carbohydrates, also commonly referred to as “sugars,” can be represented in many forms. One such form is a Fischer projection, a two-dimensional representation of a molecule that gives three-dimensional information. The horizontal lines of a Fischer projection are equivalent to wedges: functional groups on horizontal lines are coming out of the page. The vertical lines are equivalent to dashes: functional groups on vertical lines are going into the page.
 Fischer Projections of D- And L- Forms of Glyceraldehyde.
Fischer Projections of D- And L- Forms of Glyceraldehyde.
- For sugars, the absolute configuration is designated using D- and L- nomenclature instead of the R and S system used in organic chemistry. If the hydroxyl group of the highest-numbered chiral carbon is on the right, the sugar is in the D-configuration. If the hydroxyl group of the highest-numbered chiral carbon is on the left, it is in the L-configuration.
- While Fischer projections represent the straight-chain form of carbohydrates, you may also see sugars represented in their cyclic form as Haworth projections. In a cyclic sugar, the anomeric carbon is the carbon that has two bonds to oxygen.
- Be aware that a cyclic sugar can exist in two possible anomers: an ⍺-anomer and a β-anomer. A sugar is in its α-anomer form when the anomeric carbon’s substituent is on the opposite side of the plane as the highest numbered chiral center’s substituent. A sugar is in its β-anomer form when the anomeric carbon’s substituent is on the same side of the plane as the highest numbered chiral center’s substituent.
 Haworth Projections of the Α- And Β-Anomers of D-Glucose.
Haworth Projections of the Α- And Β-Anomers of D-Glucose.
(b) Cyclization
- Cyclic sugars are formed via an intramolecular reaction, or a reaction between two functional groups on the same molecule. Aldoses are sugars with an aldehyde group (-CHO). Their cyclization forms a hemiacetal. On the other hand, Ketoses have a ketone group (-CO) and no other further oxidized functional groups. Their cyclization forms a hemiketal.
- It’s easiest to visualize this reaction when the carbohydrate is drawn in its linear form (e.g., in a Fischer projection). During cyclization of both aldoses and ketoses, the hydroxyl group (nucleophile) on the highest-numbered chiral center attacks the carbonyl group (electrophile).
 Cyclization of D-Fructose, A Hemiketal. The Second Carbon is Both the Site of Nucleophilic Attack and The Anomeric Carbon (As It Is Bonded To 2 Oxygen Atoms).
Cyclization of D-Fructose, A Hemiketal. The Second Carbon is Both the Site of Nucleophilic Attack and The Anomeric Carbon (As It Is Bonded To 2 Oxygen Atoms).
Note that as we convert the structure of the sugar from its two-dimensional to three-dimensional structure, the functional group that is pointing to the right of the Fischer projection will end up pointing downward in the Haworth projection.
- For most sugars that are dissolved in a solution, this cyclization occurs spontaneously. As a result, many sugars can be found in various combinations of D-, L-, and linear forms. (The final ratios of each of these forms are usually affected by stereochemistry unique to each molecule.) This tendency to undergo spontaneous cyclization is known as mutarotation.
- Sugars can also be described as being “non-reducing” or “reducing.” A reducing sugar is one that can act as a reducing agent. Reducing sugars can be identified through the presence of a free anomeric carbon, meaning it is not in a glycosidic bond and has a free hydroxyl group. Conversely, non-reducing sugars lack a free anomeric carbon.
- Benedict’s reagent and Tollen’s reagent are two reagents that are commonly used to detect the presence of reducing sugars. The two reagents react with reducing functional groups in unique ways: Benedict’s reagent reacts with aldoses to form a red copper precipitate, while Tollen’s reagent reacts with aldehydes to form a silver, mirrorlike precipitate.
Sugar Structures
A sugar monomer is referred to as a monosaccharide. Two monosaccharides can be attached via a glycosidic linkage to form a disaccharide. Multiply monomers that are linked together form a polysaccharide.
All monosaccharides are considered reducing sugars. Sucrose, a disaccharide we will discuss below, is a common example of a non-reducing sugar.
All monosaccharides, regardless of form, share a common chemical composition:
- One part carbon atoms
- Two parts hydrogen atoms
- One part oxygen atoms
This composition varies slightly in disaccharides and polysaccharides but holds true for the composition of all monosaccharides.
The MCAT will test your knowledge of how these sugars behave in reactions and also on the structure of particular monosaccharides and disaccharides. Some of these structures are discussed below.
(a) High-yield Monosaccharides
One of the most common monosaccharides you will encounter is glucose. This is an aldose sugar, and in its cyclic form, it is classified as a pyranose, meaning it is a six-membered ring.
Another common sugar you may see is fructose. This monosaccharide is a ketose sugar, and in its cyclic form, it is classified as a furanose, meaning it is a five-membered ring. (You may also see certain sugars referred to as hexoses and pentoses, meaning they have six and five carbon atoms, respectively. Note that it’s possible for a sugar to be both a hexose and a furanose—the sugar would simply have five atoms within the ring and at least one carbon atom outside of the ring.)
Galactose, like glucose, is another aldose sugar. However, this sugar has four cyclic isomers, with two being pyranoses and the other two being furanoses. Study These Monosaccharides Well! You Should Expect to Be Tested on Them.
Study These Monosaccharides Well! You Should Expect to Be Tested on Them.
- Oxyribose, or ribose, is a monosaccharide that is a constituent of RNA. Deoxyribose, a relative of ribose, is a constituent of DNA. A deoxy sugar is a ribose that has replaced one hydroxyl group with a hydrogen atom. In RNA, the 2’ carbon has a hydroxyl substituent, whereas in DNA, the 2’ carbon has this replaced with a hydrogen atom.
 Ribose And Deoxyribose Differ by One Hydroxyl (-Oh) Group.
Ribose And Deoxyribose Differ by One Hydroxyl (-Oh) Group.
You should be able to recognize and differentiate the structures of the pentose sugars found in DNA and RNA.
(b) High-yield disaccharides
In addition to the above monosaccharides, it will be useful to memorize the structures of certain disaccharides. Here’s a helpful hint: these disaccharides will be formed by combinations of the monosaccharides you already know! Recall that monosaccharides can be attached to each other through glycosidic bonds.
- Sucrose is formed via the α-1,2 glycosidic linkage of an α-glucose molecule and a β-fructose molecule. This means that an α-bond is formed between the 1st and 2nd carbons of glucose and fructose, respectively.
- Lactose is formed via the β-1,4 glycosidic linkage of a β-galactose and β-glucose
- Maltose is formed via the α-1,4 glycosidic linkage of two α-glucose molecules.
 The Structures of High-Yield Disaccharides.
The Structures of High-Yield Disaccharides.
(c) Glycogen
- While monosaccharides and disaccharides are the most usable form of energy, they can be highly inefficient to store. As a result, our bodies store carbohydrates in the form of a polysaccharide known as glycogen.
- Glycogen is a polysaccharide that is highly branched and compacted—to maximize storage capacity and minimize the solvation energy needed to store this molecule. (For a further discussion on solvation energy, refer to our guide on proteins.)
- Starting with a peptide core, an enzyme known as glycogen synthase linearly arranges the glucose molecules via α-1,4 glycosidic links. Another enzyme known as branching enzyme hydrolyzes one of these linkages and starts a new branch using the oligosaccharide and attaching it via an α-1,6 glycosidic linkage. Multiple glycogen chains are arranged in a spherical fashion to form a granule. This process of glycogen formation is known as glycogenesis.
- In the reverse process, known as glycogenolysis, the large glycogen molecule is broken down into its smaller glucose units. An enzyme known as glycogen phosphorylase breaks the α-1,4 glycosidic linkages in a linear chain until it reaches the branching point. Here, the debranching enzyme will hydrolyze the α-1,4 glycosidic linkage and relocate the oligosaccharide to the end of another linear chain. It will then hydrolyze an α-1,6 glycosidic linkage between the branched glucose molecule and the linear chain.
- Note that two enzymes are required for both glycogenesis and glycogenolysis: one to create/break down glucose molecules in a linear chain, and another to branch/debranch a chain of existing glucose molecules.
- For further information on the fate of glucose and its pathways, refer to our guide on carbohydrate metabolism.
High-Yield Terms
- Fischer projection: a two-dimensional representation of a molecule that gives three-dimensional information; represents sugars in D- and L-configurations
- Haworth projection: a representation of the cyclic forms of sugars; represents sugar in their ⍺-anomer or β-anomer form
- Aldose: a sugar with an aldehyde group (-CHO); cyclization forms a hemiacetal
- Ketose: a sugar with a ketone group (-CO); cyclization forms a hemiketal
- Mutarotation: the ability of monosaccharides in aqueous solution to undergo spontaneous cyclization
- Pyranose: a sugar that contains a six-membered ring; e.g., glucose
- Furanose: a sugar that contains a five-membered ring; e.g., fructose
- Benedict’s reagent: reacts in the presence of aldoses to form a red copper precipitate
- Tollen’s reagent: reacts in the presence of aldehydes to form a silver, mirrorlike precipitate
- Glycogen: a polysaccharide used by the body as its primary form of carbohydrate-based energy storage
Passage-Based Questions and Answers
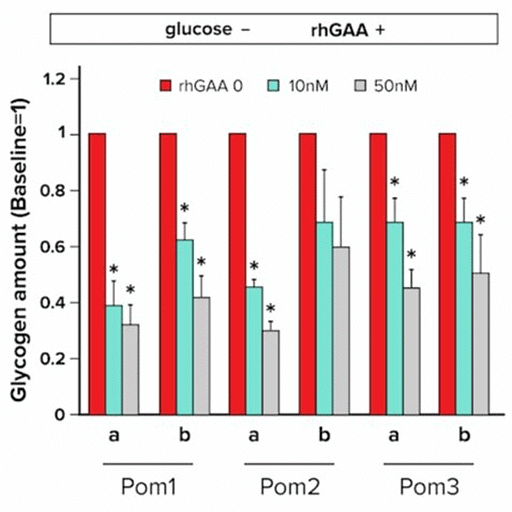
Congenital defects in metabolic pathways can result in chronic deficiencies that persist through infancy and adulthood. A congenital deficiency of the metabolic enzyme α-glucosidase (GAA) leads to infantile-onset Pompe disease (IOPD). This disease results in the buildup of lysosomal glycogen in tissues such as the skeletal muscle and heart, along with risk of heart failure in early infancy.
Glycogen is typically located in the cell cytoplasm, with only a small amount of glycogen present in the lysosomes. In one experiment, researchers collected and analyzed myocytes derived from the pluripotent stem cells of three IOPD patients (Pom1, Pom2, Pom3) to investigate the pathophysiology of the disease and possible therapeutic interventions. A glucose-free culture medium was used to establish cell cultures. A periodic acid-Schiff stain was conducted to compare the amounts of glycogen in IOPD-derived myocytes and control. The results are shown in Figure.
 CTR - Control; Pom - Experimental Condition
CTR - Control; Pom - Experimental Condition
Enzyme replacement therapy with recombinant human GAA (rhGAA) has been shown to improve the survival rate in IOPD patients. In a second experiment, researchers added rhGAA at three different concentrations to 3 previously established cell cultures and recorded the amount of glycogen present in cells. The results are shown in Figure.
|
73 videos|18 docs|7 tests
|

|
Explore Courses for MCAT exam
|

|
















