Overview: Metals & Non-metals - 4 | Advance Learner Course: Science Class 9 PDF Download
Occurrence of Metals
Elements or compounds which occur naturally in the earth’s crust are known as minerals. Minerals from which metals can be extracted profitably are called ores. Seawater also contains some soluble salts such as sodium chloride, magnesium chloride, etc.
Extraction of Metals
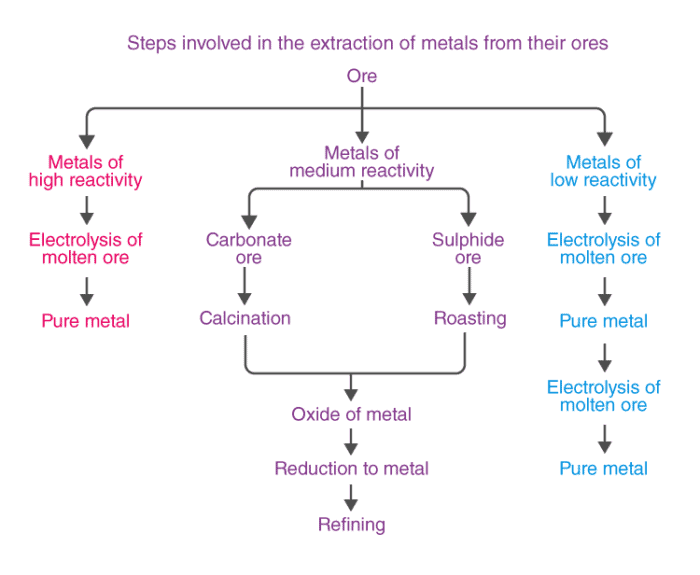
On the basis of reactivity, we can group the metals into three categories :
(i) Metals of low reactivity. For example, gold, silver, platinum and copper.
(ii) Metals of medium reactivity. For example, zinc, iron, lead, etc.
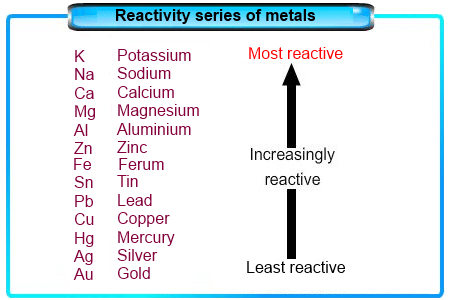
(iii) Metals of high reactivity. For example, potassium, sodium, calcium, magnesium and aluminium.
- Ores of many metals are oxides. This is because oxygen is a very reactive element and is very abundant.
- Different techniques are to be used for obtaining the metals falling in each category Several steps are involved, in the extraction of pure metal from the ores.
Enrichment of ores
Enrichment of ores
- Ores are generally contaminated with impurities, such as soil, sand, etc., called gangue.
- These impurities must be removed from the ores before proceeding to extraction.
- The method used for removing gangue depends upon the physical and chemical properties of the gangue and the ore.
Extracting metals low in the activity series
Extracting metals low in the activity series
- Such metals are very unreactive. For example, cinnabar (HgS) is an ore of mercury.
- When heated, it is first converted to mercury oxide which on further heating changes to metallic mercury.
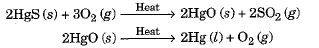
- Similarly, copper which occurs in nature as Cu2S can be obtained by just heating in air.
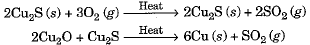
Extracting metals in the middle of the activity series
Extracting metals in the middle of the activity series
- Such metals (Fe, Zn, Pb, Cu, etc.) are usually present in the form of sulphides and carbonates, which are both converted into oxide first.
- The sulphide ore is converted into oxide ore by heating strongly in the presence of excess air. This process is known as roasting.
- The carbonate ore is changed into oxide by heating strongly in limited supply of air.
Calcination
The metal oxides are then subjected to reduction with carbon.

- Reduction can also be brought about by reactive metals like sodium, calcium, aluminium etc. When manganese dioxide is heated with aluminium, the following reaction takes place:
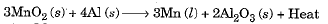
- In the above reaction, Mn is getting reduced while Al is getting oxidised. Al is higher on the activity series than Mn.
- The amount of heat evolved is so large that the metal is obtained in the molten state.
Thermit Process
- The reaction of iron (III) oxide (Fe2O3) with aluminium is an exothermic process. Iron is obtained in molten state which is used to join railway tracks or cracked machine parts.
- This reaction is known as thermit reaction and the process is known as thermit process.
Extraction of Metals towards the top of Activity Series
- The metals at the higher end of the activity series are very reactive.
- Their oxides cannot be reduced with carbon because the metals have greater affinity for oxygen than carbon.
- Such metals are obtained by electrolytic reduction. Sodium, magnesium and calcium are obtained by the electrolytic reduction of their molten chlorides. The metals are obtained at the cathode while chlorine is liberated at the anode.
- The reactions may be represented as :
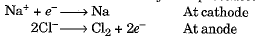
Aluminium is obtained by electrolytic reduction of aluminium oxide.
Refining of Metals
Electrolytic Refining
- Metals such as copper, zinc, tin, nickel, silver, gold, etc., are refined electrolytically. In this process, impure metal is made anode and a thin strip of pure metal is made the cathode.
- A solution of metal salt is used as the electrolyte. The apparatus is shown in the figure below.
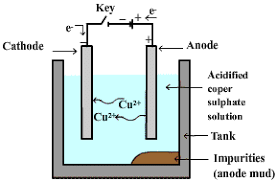 Electrilytic refining of Copper
Electrilytic refining of Copper - The electrolyte is a solution of acidified copper sulphate.
- The anode is impure copper, whereas, the cathode is a strip of pure copper.
- On passing electric current, pure copper is deposited on the cathode.
- On passing the current, the pure metal from the anode dissolves into the electrolyte.
- An equivalent amount of pure metal from the electrolyte is deposited on the cathode.
- Insoluble impurities settle down at the bottom of the anode and are known as anode mud.
Corrosion
- Silver articles turn black when exposed to air. This is because it reacts with hydrogen sulphide in air to form silver sulphide which is black.
- Similarly copper articles turn green due to reaction with atmospheric carbon dioxide form in g green copper carbonate.
- Iron gets rusted when exposed to moist air form brown oxide of iron.
These are all examples of rusting.
Activity II. Perform an experiment to find out conditions which cause rusting of iron. Materials required: Three test tubes with corks, iron nails, anhydrous calcium chloride, oil.
Procedure:
- Take three test tubes. Place iron nails in each of them.
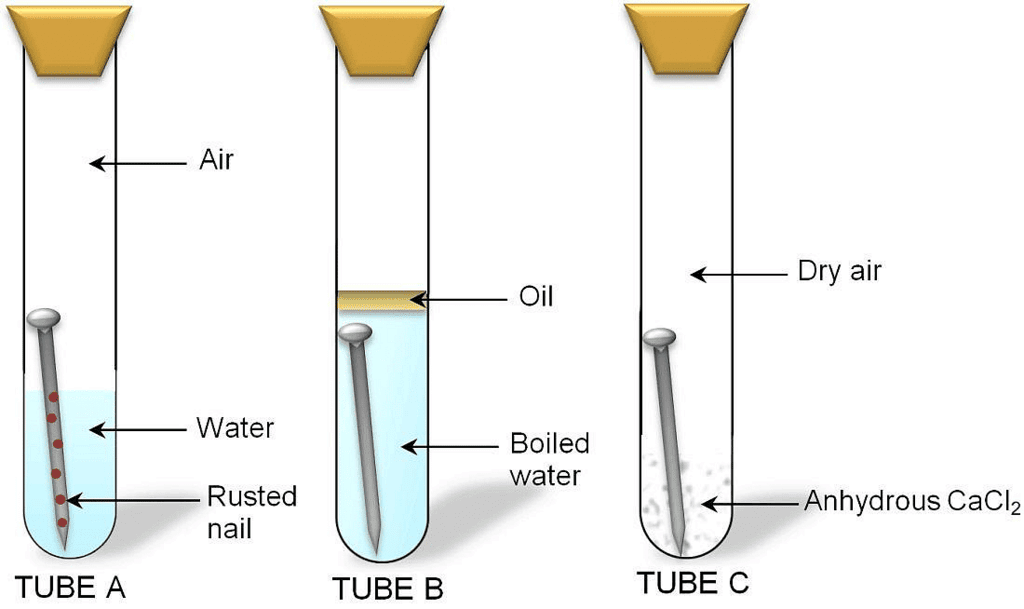 Investigating the condition under which iron rusts
Investigating the condition under which iron rusts - Label these tubes A, B and C.
- Pour some water in test tube A and cork it.
- Pour boiled water in test tube B. Add 1 mL of oil and cork it. The oil will form the upper layer and prevent the air from dissolving in water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture from the air.
- Leave these test tubes for a few days.
It is observed that iron nails rust in test tube A but they do not rust in test tubes B and C. This is because iron nails are exposed to both water and air in test tube A. In test tube B, the nails are exposed to only water and in test tube C, the nails are exposed to only air. This means presence of both water and air necessary for rusting to take place.
Prevention of Corrosion
Prevention of Corrosion
Rusting of iron can be prevented by the following methods:
- By painting the surface.
- By oiling or greasing the surface.
- Galvanizing, chrome plating, anodising or making alloys.
Galvanisation
Galvanisation
- It is a method of protecting steel and iron from rusting. The article is coated with a thin layer of zinc. Galvanised article is protected against rusting even if zinc coating is broken.
Alloying
Alloying
It is a method of improving the properties of a metal. If iron is mixed with a small amount of carbon (about 0.05 %), it becomes hard and strong.
- When iron is mixed with nickel and chromium, we obtain stainless steel which is hard and does not rust.
- An alloy is a mixture of two or more metals, or a metal and a non-metal.
- It is prepared by first melting the primary metal and then dissolving the other elements in a definite proportion.
- It is then cooled at room temperature.
22-carat gold
22-carat gold
- Pure gold which is 24-carat gold is very soft and is not suitable for making ornaments.
- It is alloyed with either copper or silver to make it hard.
- Generally, in India, 22-carat gold is used for making jewellery, it means 22 parts of gold is alloyed with 2 parts of copper or silver.
Properties of alloys
Properties of alloys
- Alloy of a metal with mercury is known as amalgam. It has been observed that melting point and electrical conductivity of an alloy are lower than those of the constituent metals.
- For example, brass which is an alloy of copper and zinc, and bronze which is an alloy of copper and tin are not as good conductors of electricity as copper.
Solder which is an alloy of lead and tin has a low melting point and is used for welding electrical wires. - The iron pillar near the Qutab Minar was build more than 1600 years ago. The iron workers of India at that time had developed a technique which prevented rusting of iron.
- Scientists from all parts of the world have checked the quality and found that the technique used is really rust resistant i.e., it resists the formation of rust. The pillar has a height of 8 m and weight 6 tonnes or 6,000 kg.
|
12 videos|50 docs|17 tests
|
FAQs on Overview: Metals & Non-metals - 4 - Advance Learner Course: Science Class 9
| 1. What is the process of extraction of metals? |  |
| 2. How is the enrichment of ores carried out? |  |
| 3. What is calcination and how is it used in the extraction of metals? |  |
| 4. How is corrosion prevented? |  |
| 5. What is the thermit process? |  |
















