Group 16 Elements: Oxygen Family | Inorganic Chemistry PDF Download
GROUP 16 (OXYGEN FAMILY)
(1) ATOMIC & PHYSICAL PROPERTIES
| Element | O | S | Se | Te |
| Atomic Number | 8 | 16 | 34 | 52 |
| Atomic Mass | 16 | 32.06 | 78.96 | 122.6 |
| Electronic configuration | (He)2s2 3p4 | (Ne) 3s2 3p4 | [Ar] 3d10 4s2 4p4 | [Kr] 4d10 5s2 5p4 |
| Covalent Radius/pm | 74 | 103 | 119 | 142 |
| Ionization enthalpy (kJ mol–1) | 1314 | 133 | 941 | 869 |
| Electronegativity | 3.5 | 2.44 | 2.48 | 2.01 |
| Boiling Point/K | 90 | 718 | 958 | 1260 |
(2) ABUNDANCE IN THE EARTH CRUST: O > S > Se > Te
(3) PHYSICAL STATE
Oxygen is gas while other are solids at room temperature. Oxygen exists as diatomic molecule where as other elements e.g. sulphur exists as shown into the following crown shape (puckered ring structure S8)
(4) METALLIC ACID NON-METALLIC CHARACTER
Metallic character increases with increase in atomic number.
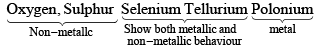
(5) OXIDATION STATES
- Oxygen shows-2 oxidation state in most of its compounds (being highly electronegative). Exception, in OF2 & O2F2 oxidation state of oxygen is +2 and +1
- Sulphur shows –2, +2, +4 and +6 oxidation states. Availability of +4 and + 6 oxidation states are due to the presence of empty d-orbitals.
- Sulphur shows higher oxidation states with compounds of oxygen and fluorine (h ighly electronegative elements( (SO2, SO3, SF4, SF6)
- The tendency to show –2 oxidation state decreases down the group since the electronegativity decreases.
(A) Oxygen (O)
If differs from the remaining element of the VIth group because of the following properties (A) Small Size (B) High electronegativity (C) Non-availability of d-orbitals.
(i) Preparation
1. 2HgO 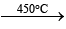 2Hg + O2;
2Hg + O2;
2Ag2O 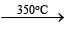 4Ag + O2
4Ag + O2
2. 2NaNO3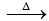 2NaNO2 + O2;
2NaNO2 + O2;
2KClO3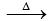 2KCl + 3O2 (laboratory method)
2KCl + 3O2 (laboratory method)
3. 4K2Cr2O7 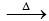 4K2CrO4 + 2Cr3O3 + 3O2
4K2CrO4 + 2Cr3O3 + 3O2
2KMnO4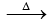 K2 MnO4 + MnO2 + O2
K2 MnO4 + MnO2 + O2
(ii) Physical Properties
Colourless, odourless and tasteless gas. It is paramagnetic and exhibits allotropy. Three isotopes of oxygen are 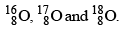 Oxygen does not burn but is a strong supporter of combustion.
Oxygen does not burn but is a strong supporter of combustion.
(iii) Chemical Properties
(a) OXIDES
(a-i) Acidic oxides.
Oxides of Non-metals and metals in highest oxidation state.
They dissolve in water forming oxyacids. e.g. CO2, SO2, N2O3, P4O6, P4O10, Cl2O7, CrO3, Mn2O7 etc.
Ex.: Cl2O7 + H2O → 2HClO4;
SO3 + H2O → H2SO4
(a-ii) Basic oxides (Oxides of metals)
They either dissolve in water to form alkalies or combine with acids to form salts and water or combine with acidic oxides to form salts.
Li2O, Na2O, BeO, MgO, CaO, SrO, BaO, CuO, FeO etc.
Na2O + H2O→2NaOH
(a-iii) Natural Oxides (Oxides of Non metals)
The neither combine with acids nor with the bases to form salts e.g. CO, N2O, NO, H2O etc.
(a-iv) Amphoteric oxides (Metal oxides)
These can combine with acid as well as bases e.g. ZnO, Al2O3, BeO, Sb2O3, Cr2O3, PbO, PbO2, etc.
PbO + 2NaOH→Na2PbO2 + H2O;
PbO + H2SO4 →PbSO4 + H2O
(a-v) Peroxides (Metal oxides)
They react with dil. Acids and form H2O2, e.g. Na2O2, K2O2, BaO2 etc.
Na2O2 + H2SO4→Na2SO4 + H2O2
(a-vi) Dioxides (metal oxides)
Like peroxide they contain excess of oxygen but do not yield H2O2 with dil. Acids e.g. PbO, MnO2 etc. They evolve Cl2 with conc. HCl and O2 with conc. H2SO4.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
(a-vii) Super Oxides (metal oxides)
They contain O2– ion, e.g. KO2, RbO2 and CsO2. These oxides react with water forming H2O2 and O2 2KO2 + 2H2O → 2KOH + H2O2 + O2
(a-viii) Sub Oxides
They contain less oxygen than expected from the normal valency of the elements e.g. C3O2, N2O, Pb2O, Hg2O etc.
C3O2 → O=C=C=C=O
(b) OZONE (O3)
(i) Preparation
It is prepared by passing silent electric discharge through pure and dry oxygen
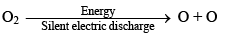
O2 + O →O3;
ΔH = 245 kJ mol-1
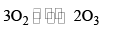
(ii) Properties
(a) Oxidising agent: Ozone is a strong oxidizing agent in acidic medium
O3 + 2H+ + 2e–→ O2 + H2O; SRP = + 2.07 V (acidic medium)
O3 + H2O + 2e→O2 + 2OH; SRP = + 1.24 V (alkaline medium)
O3→O2 + [O]
E.g.
1. 2KI + H2O + [O]→2KOH + O2 + I2
2. KI + 3O3→KIO3 + 3O2
3. S + 3O3 + H2O→H2SO4 + 3O2
4. 2I2 + 9[O3]→I4O9 + 9O2
- I2O9 yellow solid has the composition I+3 (IO–3)3. Formation of this compound is a direct evidence in favour of basic nature of I2 (i.e. its tendency of form cations)
O3 + H2O2→2O2 + H2O
5.Oxidising Redusing
agent agent
TAILING OF MERCURY
Pure mercury is mobile liquid but when brought in contact with O3 its mobility decreases and it starts sticking to glass surface forming a type of tail due to the dissolution of Hg2O (mercury suboxide) in Hg.
- O3 is used as a germicide and disinfectant for sterilizing water and improving the atmosphere of crowded places.
HYDROGEN PEROXIDE (H2O2)
(i) Preparation
(1) BaO2.8H2O + H2SO4(cold)→BaSO4 ↓ (white) + H2O2 + 8H2O
2) H2S2O8 + H2O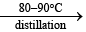 H2SO4 + H2SO5
H2SO4 + H2SO5 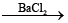 BaSO4 ↓ (white) + H2O2(aq)
BaSO4 ↓ (white) + H2O2(aq)
Comparison (H2O & H2O2)
(i) Its boiling point 144 °C more than water but freezing point ( –4) less than water. Density a nd dielectric constant are higher than H2O.
(ii) Its aqueous solution is more stable than the anhydrous liquid where it decomposes into water and O2.
2H2O2→2H2O + O2
H2O2 is not kept in glass containers because traces of alkali metal ions from the glass can catalyzed the explosive decomposition of H2O2. Therefore, aqueous solutions is stored in plastic containers and some urea or phosphoric acid or glycerol is added to that solution because these compounds have been found to behave as negative catalyzed for the decomposition of H2O2.
(iii) Acidic nature
Behaves as a weak acid according to the following equation
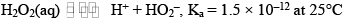
Aqueous solution of H2O2 turns blue litmusred which is then bleached by the oxidizing property of H2O2.
Na2CO3 + H2O3→Na2O2 + H2O + CO2
Ba(OH)2 + H2O2 + 6H2O→BaO2.8H2O↓
A 30% H2O2 solution has pH = 4.0
(iv) Oxidizing Agent
2e + 2H+ + H2O2→2H2O; SRP = 1.77 v (in acidic medium)
2e + H2O2 →2HO–; SRP = 0.87 v (In alkaline medium)
On the basis of the above potentials, we can say that H 2O2 is strong oxidizing a gent in acidic medium but kinetically its is found that reactions are faster in basic medium.
Cr2O72– + 2H+ + 4H2O2→2CrO5 + 5H2O
CrO5 bright blue coloured compound is soluble in ether.
Uses
(i) In bleaching of delicate materials such as silk, wool, cotton, ivory etc.
Properties of Hydrides
Table: Some Properties of H2O, H2S, H2Se and H2Te
| Enthalpies of formation (kJ mol–1) | Bond angle | Boling Point (C°) | |
| H2O | –242 | H—O—H = 108°28’ | 100 |
| H2S | –20 | H—S—H = 92° | –60 |
| H2Se | +81 | H—Se—H = 91° | –42 |
| H2Te | +154 | H—Te—H = 90° | –2.3 |
The hydrides decreases in stability from H2O to H2Te. They become less stable because the bonding orbitals become larger and more diffuse hence overlap with the hydrogen ‘1s orbital’ is less effective.
The H—O—H bond angle in water is 104°28’, in accordance with the VSEPR prediction of slightly less than tetrahedral due to the presence of lone pairs of electrons. Thus the orbitals used for bonding by O are close to sp3 hybrids. In H2S to H2Te the bond angles become close to 90°.this suggests that almost pure p orbitals on Se and Te are used for bonding to hydrogen. In a series of similar compounds, the boiling points usually increase as the atoms become larger and heavier. If the boiling points increase, then the volatility decrease. This trend is shown by the boiling points of H2S. H2Se & H2Te, but the boiling point of water is anomalous.
Water has an abnormally low volatility because its molecules are associated with each other by means f hydrogen bonds in both the solid and liquid states.
SULPHUR (S)
Sodium Thiosulphate (Na2S2O3.10H2O): (Hypo)
Preparation
(i) Na2SO3S  Na2S2O3
Na2S2O3
Na2 SO3+ 2SO2 (excess)+ H2O→2NaHSO3+ CO ;
(ii) 2NaHSO3+ Na2CO3→2Na2S2O3+ H2O +CO2
Structure of Oxo acids of S
1. Sulphurous acid series
| H2SO3 Sulphurous acid | 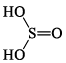 | S(+IV) |
| H2S2O5 pyrosulphurou s acid | 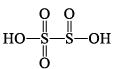 | S(+V), S(+III) |
| H2S2O4, dithionous acid | 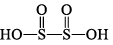 | S(+III) |
2. Sulphuric acid series
H2SO4 Sulphuric acid | 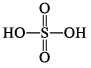 | S(+IV) |
| H2S2O3 thiosulphuric acid | 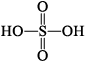 | S(+VI), S(–II) |
| H2S2O7 di or pyrosulphuric acid | 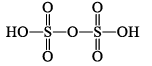 | S(+VI) |
3. Thionic acid series
| H2S2O6 dithionic acid | 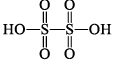 | S(+VI) |
| H2SnO6 polythionic acid (n = 1 – 12) | 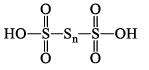 | S(+V) S(0) |
4. Peroxoacid series
| H2SO5 peroxomonosulphuric acid | 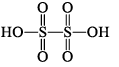 | S(+VI) |
| H2S2O8 peroxodisulphuric acid | 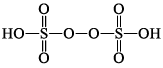 | S(+VI) |
|
48 videos|92 docs|41 tests
|
FAQs on Group 16 Elements: Oxygen Family - Inorganic Chemistry
| 1. What are the elements in the Oxygen family? |  |
| 2. What are the common properties of the Oxygen family elements? |  |
| 3. How does the reactivity change within the Oxygen family? |  |
| 4. What are some common uses of the Oxygen family elements? |  |
| 5. How do the physical properties vary within the Oxygen family? |  |

|
Explore Courses for Chemistry exam
|

|


















