Group 17 Elements: Halogen Family | Inorganic Chemistry PDF Download
GROUP 17 (VIIA-THE HALOGEN FAMILY
Physical Properties of the Halogens
| S. No. | Properties | F | Cl | Br | I |
| 1. | Atomic Number | 9 | 17 | 35 | 153 |
| 2. | Electronic configuration | (He)2s22p5+ | (Ne)3s23p5 | [Ar] 3d104s2 | [Kr] 4d105s2 |
| 3. | Atomic radius/pm | 72 | 99 | 114 | 133 |
| 4. | (IE)I/kJ mol–1 | 1681 | 1256 | 1143 | 1009 |
| 5. | Electronegativity (Pauling) | 4.0 | 3.0 | 2.8 | 2.5 |
| 6. | Bond energy (XX)kJ mol–1 | 126 | 210 | 158 | 118 |
| 7. | Oxidising power (X2) | Most | - | - | Least |
| 8. | Common oxidation state | -1 | –1, +1, +3, +5, +7 | –1, +1, +3, – 1, +5, +1, +3, +7 | +5, +1 |
| 9. | Physical form (X2) at room temperature pale | Yellow gas | Yellow-green gas | Dark red liquid | Violet-black solid |
| 10. | Melting point (X2)/°C | –219 | –101 | –7 | 114 |
- HF has a high b.p. (19.5 °C) as a result of strong intermolecular hydrogen bonding, whereas all other hydrogen halide have much lower b.p.
- HF is a weak acid, whereas all other hydrohalic acids (HCl, HBr and HI) are strong acids.
- F2 reacts with cold NaOH solution to produce OF2 (oxygen diflu oride) gas 2F2 + 2NaOH → 2NaF + H2O + OF2 The same reaction with chlorine or bromine produces a halides (X–) and hypohalite (XO) X2 + 2NaOH → NaX + NaXO + H2O X stands for Cl or Br, Iodine (I2) does not react under the same condition
- Ca(OH)2 + Cl2 →CaCl(OCl) + H2O
- Hot NaOH 6NaOH + 3X2→ 5NaX + NaXO3 + 3H2O (X = Cl, Br, I)
Reactions of Halogen
| Compound | Reaction | Comment |
| H2O | 2F2 + 2H2O →4H+ + 4F– + O2 2X2 + 2H2O → 4H+ + 4X– + O2 X2 + H2O | Vigorous reaction with F2 Atmospheric O2 can oxidize I– to I2 hence, reverse rea ction Cl2 > Br2 > I2 (F2 does not disproportionate) All the halogens with Br2 photo chemical reaction, with I2 very slow even at high temperature. |
| H2 | H2 + X2 → 2MXn | Most metals form halides. |
| CO | CO + X2 → COX2 | Only Cl2, Br2 form carbonyl halide |
| P | 2P + 3X2 → 2PX3 2P + 5X2 → 2PX5 | For F, Cl, Br, I For F, Cl, Br |
| S | 2S + X2 → S2X2 S + 2Cl2 → SCl4 S + 3F2 → SF6 | Cl, Br |
| H2S | H2S + X2 → 2HX + S | All the halogens oxidize H2S(S2–) to S |
| SO2 | SO2 + X2 → SO2X2 | F and Cl |
| NH3 | 8NH3 + 3X2 → N2 + 6NH4X | F, Cl, Br |
| X2 | X2 + X’2 → 2XX’ | Interhalogen compound |
Compounds of Halogens With Oxygen
| Compound | Preparation | Structure | Properties |
| 1. Oxides of fluorine | |||
| (a) IF2 | 2F2 + 2NaOH →2NaF + H2O + OF2 | 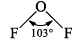 | Strong fluorinating agene |
| (b) O2F2 | F2 + O2 O2F2 O2F2 | Similar to H2O2 | Strong fluorinating agene |
| 2. Oxides of chlorine | |||
| (a) Cl2O | 2Cl2 + 2Na2CO3 + H2O→Cl2O + 2NaHCO3 + 2NaCl | 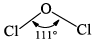 | Explosive in nature |
| (b) ClO2 | 2AgClO3 + Cl2 → 2AgCl + 2ClO2 + O2 2KClO3 + 2(COOH)2 → 2ClO2 + 2CO2 + (COOK)2 + 2H2O |  | Odd electron molecule but do not dimerise because odd electron is delocalize |
| (c) Cl2O6 | 2ClO2 + 2O3 → 2ClO3 + 2O2 → Cl2O6 + 2O2 | 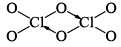 | Diamagnetic in nature |
Pseudohalogens and Pseudohalides
A few ions are known consisting of two or more atoms of which at least one is N that have properties similar to those of the halide ions. They are therefore called pseudohalide ions. Pseudohalide ions are univalent and these form salts resembling the halide salts. For example the sodium salts are soluble in water, but the silver salts are insoluble. The hydrogen compounds are acids like the halogen acids HX.
Table: The important pseudohalogens
| Anion | Acid | Dimer |
| CN– cyanide ion SCN– thiocyanate ion SeCN– selenocyanate ion OCN– cyanate ion NCN2– cyanamide ion ONC– fulminate ion N3– azide ion | HCN hydrogen cyanide HSCN thiocyanic acid HOCN cyanic acid H2NCN cyanamide HONC fulminic acid HN3 hydrogen azide | (CN)2 cyanogen (SCN)2 thiocyanogen (SeCN)2 selenocyanogen |
Some of the pseudohalide ions combine to form dimers comparable with the halogen molecules X2. These include cyanogens (CN)2, thiocyanogen (SCN)2 and selenocyanogen (SeCN)2. The best known pseudohalide is CN–. This resembles Cl–, Br– and I– in the following respects.
1. It forms an acid HCN
2. It can be oxidized to form a molecule cyanogens (CN)2.
3. It forms insoluble salts with Ag+, Pb2+ and Hg+
4. Interpseudohalogen compounds ClCN, BrCN and ICN can be formed.
5. AgCN is insoluble in water but solube in ammonia, as is AgCl.
6. It forms a large number of complexes similar to halide complexes. e.g. [Cu(CN)4]2– and [CuCl4]2– and [Co(CN)6]3– and [CoCl6]3–
GROUP 18TH ELEMENTS (ZERO GROUP FAMILY)
ATOMIC & PHYSICAL PROPERTIES
| S.No. | Element | He | Ne | Ar | Kr | Xe |
| 1 | Atomic Number | 2 | 10 | 18 | 36 | 54 |
| 2 | Atomic mass | 4 | 20.18 | 39.1 | 83.8 | 131.1 |
| 3 | Electronic configuration | 1s2 | [He] 2s22p6 | [Ne] 3s22p6 | [Ar] 3d104s24p6 | [Kr] 4d105s25p6 |
| 4 | Atomic Radius (pm) | 120 | 160 | 190 | 200 | 220 |
| 5 | Ioniza tion enthalpy (kJ mol–1) | 2372 | 2080 | 1520 | 1351 | 1170 |
| 6 | Boiling point /K | 4.2 | 27.1 | 87.2 | 119.7 | 165 |
| 7 | Electronegativity |
Initially their electronegativities were taken to be zero. But it is not zero.
1. All are gases at room temperature. The only radioactive element in them is Radon (Rn) 2. Order of abundance in the air Ar > Ne > Kr > He > Xe 3. If Helium is compressed and liquefied it forms He(l) liquid at 4.2 K. This liquid is a normal liquid like any other liquid. But if it is further cooled then He(II) is obtained at 2.2 K. Which is known as super fluid because it is a liquid with properties of gases. It climbs through the walls of the container & comes out. It has very high thermal conductivity & very low viscosity. Compounds of inert gases are of following two types:
(i) Clatherate Compounds
Intert gas molecules get trapped in the cages formed by the crystal structure water.
During the formation of ice Xe atoms will be trapped in the cavities (or cages) formed by the water molecules in the crystal structure of ice. Compound thus obtained are called clatherate compounds. There are no chemical bonds in clatherate compound.
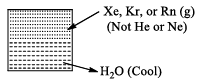
Uses of Noble, Gases
1. Uses of Helium
(i) The chief use of helium is in filling of balloons which are employed for meterologica l observations. Althoughs lifting power of helium is 8% less then that of hydrogen, yet it is preferred because it is non-inflammable.
(ii) Liquid helium (b.p. 4.2 K) is used a s a cryogenic agent for carrying out various experiments at low temperatures.
(iii) An oxygen-helium mixture is used for artificial respiration in deep sea cliving.
(iv) Helium is less soluble in blood than nitrogen. Therefore, an oxygen helium mixture is also used in die treatment of asthma.
(v) Helium is also used for creating inert atmosphere during welding of Mg and Al which are easily oxidizable.
(vi) Helium is chemically inactive and does not become radioactive. Hence it is used in gas cooled atomic reactors as a heat transfer agent.
2. Uses of Neon
(i) Neon is mainly used in discharge tubes and fluorescent lamps for advertising purpose. (ii) Neon has a remarkable property of carrying extremely high currents even under high voltage. Therefore, neon is used in safety devices for protecting electrical instruments such as voltmeters, relays and rectifiers from high voltage.
(iii) It is used in becon light a s safety signal for air nevigators since its light ha s fog penetration power.
(iv) It is used for filling sodium vapour lamps.
3. Uses of Argon
(i) Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes such as are welding of metals and alloys.
(ii) It is used in filling incandescent and flourore scent lamps where its presence retards the sublimation of the filament and thus increases the life of the lamp.
(iii) It is also used in ‘neon signs’ for obtaining lights of different colours.
4. Uses of Krypton
(i) Krypton and xenon are more efficient than argon in gas filled lamps because of their lower thermal conductivities but due to their scarcity and high cost they are used to a much smaller extent.
(ii) A mixture of krypton and xenon has also been used in some tubes for high speed photography.
5. Uses of Radon
(i) Being radioactive, radon is used in radioactive research.
(ii) It is used for normal treatment of cancer and other malignant research.
(iii) It is used in X-ray photography for the detection of flaws in metals and other solids.
COMPOUNDS OF XENON
(a) Xenon difluoride (XeF2)
(i) 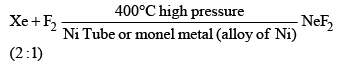
2. Xe + O2F2 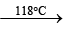 XeF2 + O2
XeF2 + O2
Reactions
1. XeF2 + H2 → Xe + 2HF
2. 2XeF2 + 2H2O → 2Xe + 4HF + O2 (Slow)
3. XeF2 + 2NaOH → Xe + 1/2 O2 + 2NaF + H2O (fast)
Oxidizing Properties
The standard reduction potential for XeF2 is measured to be +2.64 V. Therefore it acts as a strong oxidizing agent.
1. 2e + 2H+ + XeF2 → Xe + 2HF (SRP = + 2.64 V)
2. XeF2 + 2NaX → Xe + X2 + 2NaF
In this manner XeF2 will oxidize halideions (except F– into free halogens)
3. S8 + 24 XeF2 → 8SF6 + 24 Xe
4. 2CrF2 + XeF2 → 2CrF3 + Xe
5. 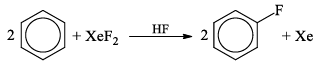
6. 8NH3 + 3XeF2 → N2 + 6NH4F + 3Xe
7. 2NO2 XeF2 → 2NO2F (Nitronium fluoride) + Xe
8. IF5 (lewis acid) + XeF2 → [XeF]+ [IF6]–; 2SbF5(Lewis acid) + XeF2 → [XeF] + [SbF6]–
(b) Xenon Tetrafluoride (XeF4)
Structure: Shape square planar & Geometry octahedral
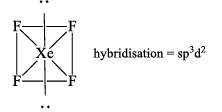
Properties
1. 6XeF4 + 12H2O → 4XeO3 + 24 HF + 3O2 + 4Xe It disproportionate into perxenate ion in basic medium.
(i) [XeO]64– XeO3 + O2
XeO3 + O2
(ii) [XeO]64– + Mn+2 → MnO4– + XeO3 (slow decomposition)
2. XeF4 + SbF5 →[XeF3]+ [SbF6]–
3. Fluorinating agent
XeF4 + Pt → PIF4 + Xe; XeF4 + 4NO→ Xe + 4NOF (nitrosyl fluoride)
XeF4 + 4NO2 ¾¾® Xe + 4NO2 F (nitronium fluoride)
4. Xenon Hexafluoride (XeF6)
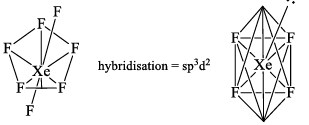
Solid XeF6 exists as tetramer in which 2[XeF5]+ ions are by F– ions
Properties
(i) HF is a good solvent for all three flu orides.
(ii) Hydrolysis
(a) Complete hydrolysis: XeF6 + 3H2O → XeO3 (white solid) + 2HF
(b) Partial hydrolysis:
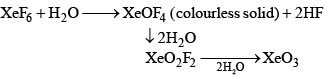
(iii) Reaction with silica (SiO2): 2XeF6 + SiO2 → 2XeOF4 + SiF4
(iv) Thermal decomposition (effect of heat): 2 XeF6 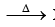 XeF2 + XeF4 + 3F2 XeF2 and XeF4 do not undergo decomposition
XeF2 + XeF4 + 3F2 XeF2 and XeF4 do not undergo decomposition
(v) Formation of addition compounds XeF6 + SbF5 → [XeF5]+ [SbF6]–; XeF6 + BF3 → [XeF5]+ [BF4]–
(vi) Reaction with H2
XeF6 + 3H2 → 6HF + Xe;
(vii) XeO3 + 2XeF6 → 3XeOF4 Order of oxidizing power XeF2 > XeF4 > XeF6
(viii) F—donating/F-accepting properties Donating XeF6 + PtF5 →(XeF5I) (PtF6–)
|
50 videos|92 docs|41 tests
|
FAQs on Group 17 Elements: Halogen Family - Inorganic Chemistry
| 1. What are the elements in Group 17 known as? |  |
| 2. What are the properties of halogens? |  |
| 3. What are some common uses of halogens? |  |
| 4. Why are halogens highly reactive? |  |
| 5. What is the trend in reactivity within the halogen family? |  |

 X– + HOX + H+
X– + HOX + H+




















