Separating Components of a Mixture, Physical and Chemical Changes & Pure Substances | Science Class 9 PDF Download
Separating the Components of a Mixture
- Many of the material around us are mixtures, these mixtures have two or more than two constituents mixed in them.
- It may not be possible to use a mixture as such in homes and in industries. We may require only one or two separate constituents of a mixture for our use.
- So, we have to separate the various mixtures into their individual constituents to make them useful in our daily life.
- The various constituents of a mixture have different physical properties such as density, solubility, size of particles volatility, boiling points, etc.
- "Heterogeneous mixtures can be separated into their respective components by simple physical methods such as handpicking, sieving, filtration etc. in everyday life."
- However, for separating homogeneous mixtures special techniques are employed depending upon the difference in one or more.
1. Separation of Coloured Components (Dye) from Blue or Black Ink
- The blue ink (or black ink) used in fountain pens is a liquid mixture. It is a mixture of a 'dye' in water.
- We can separate the coloured component (dye) by the process of evaporation. In process of separation, we do not heat the china dish containing ink directly over the flame.
- This is because the 'dye' obtained from ink can get decomposed by the high temperature produced by the direct heating with a burner. We use a 'water bath' for evaporating ink.
➢ Experiment to obtain Coloured Component (Dye) from Ink
- We take a beaker and fill it half with water (as a water bath). About 5 ml of ink (Blue ink or black ink) is put in a china dish. The china dish containing ink is then placed over the mouth of the beaker containing water, which is kept on a tripod stand.
 Ink separation using Evaporation Method
Ink separation using Evaporation Method - We now start heating the beaker with a burner, soon the water in the beaker starts boiling to form steam, this steam heats the ink in the china dish. Due to this heating, the water present in ink starts evaporating gradually.
- When all the water has evaporated from the ink. we stop heating. We will find that a small amount of solid coloured material is left in the china dish.
Thus we can separate the volatile component (Solvent) from its non-volatile solute by the method of evaporation.
2. Separation of Cream from Milk
- Sometimes the solid particles in a liquid are very small and pass through a filter paper. for such particles, the filtration technique cannot be used for separation. Such mixtures are separated by centrifugation.
➢ Centrifugation
- The method of separating finely suspended particles in a liquid, by whirling the liquid at a very high speed is called centrifugation.
 Centrifugation
Centrifugation - Principal of Centrifugation: It is based on that when a very fine suspension or a colloidal solution is whirled rapidly, the heavier particles are forced towards the bottom of the liquid and the lighter stay at the top.
- Method: Milk is a suspension of tiny droplets of oil cream in the water or liquids. The milk is put in a closed container in a big centrifugation machine. When the centrifugation machine is switched on, the milk is rotated at a very high speed in its container. Due to this, the milk separates into 'cream' and 'skimmed milk'. The cream, being lighter, floats over the skimmed milk can then be removed. Thus, the cream is separated from milk by centrifugation.
- Applications of centrifugation:
(i) It is employed in diagnostic Laboratories in testing urine and blood samples.
(ii) It is employed in blood banks to separate different constituents of blood.
(iii) It is used in drying machines to squeeze out water from wet clothes.
3. Separation of a Mixture of Two Immiscible Liquids
- The separation of two immiscible liquids is based on the difference in their densities. The apparatus used for separation is a separating funnel.
➢ Separation Through Separating Funnel
- Principle of Separating Funnel: The mixture of two immiscible liquids is put in a separating funnel and allowed to stand for some time. The mixture separates into two layers according to the densities of the liquids in it.
- Methods: Water and kerosene oil are two immiscible liquids. Pour the immiscible liquids mixture into the separating funnel. Allow the mixture to stand for half an hour or more. It forms two layers. Water being heavier forms the lower layers in the separating funnel. Whereas kerosene, being lighter, forms the upper layer.
On opening the stop-cock of separating funnel, the lower layer of water comes out first and is collected in a beaker. When the water layer has completely runoff, then the stopcock is closed. The kerosene is left behind in the separating funnel. It can be removed in a separate breaker by opening the stop-cock again. Separation of liquids
Separation of liquids - Applications:
(i) To separate mixture of oil and water.
(ii) In the extraction of iron from its ore, the lighter slag is removed from the top by this method to leave the molten iron at the bottom of the furnace.
4. Separation of a Mixture of Common Salt and Ammonium Chloride
- This method is used in the separation of such solid-solid mixtures where one of the components sublimes on heating.
- However, it is useful only if the components of the mixture do not react chemically on heating. So, we can separate ammonium chloride from a mixture of common salt and ammonium chloride by this process.
- Method: The mixtures of common salt and ammonium chloride are taken in a china dish and placed on a tripod stand. The china dish is covered with an inverted glass funnel. A loose cotton plug is put in the upper, open end of the funnel to prevent the ammonium chloride vapours from escaping into the atmosphere.
 Separation of common salt and Ammonium ChlorideThe china dish is heated by using a low Bunsen flame, on heating the mixture ammonium chloride changes into white vapour. These vapours rise up and get converted into solid ammonium chloride on coming in contact with the cold, inner walls of the funnel.
Separation of common salt and Ammonium ChlorideThe china dish is heated by using a low Bunsen flame, on heating the mixture ammonium chloride changes into white vapour. These vapours rise up and get converted into solid ammonium chloride on coming in contact with the cold, inner walls of the funnel.
When the mixture gives off no more white fumes, lift the funnel, scrap the fine white powder from its sides on a piece of paper. This is pure ammonium chloride. The residue left behind in the funnel is sodium chloride. - Some examples of solids that sublime are camphor, naphthalene and anthracene.
5. Separation of Coloured Constituents present in a mixture of Ink and Water
➢ Chromatography
- The process of separation of different dissolved constituents of a mixture by adsorbing them over an appropriate adsorbent material is called Chromatography.
- Kroma in Greek means colour. The adsorbent medium is generally magnesium oxide, alumina or filter paper.
- There are many types of chromatography but the simplest form is paper chromatography. This separation is based on the fact that the different constituents of a mixture get adsorbed differently on the same adsorbent material because they have different rates of movement. The rate of movement of each adsorbed material depends upon.
- The relative solubility of the constituent of a mixture in a given solvent. The relative affinity of the constituents of the mixture for the adsorbent medium. Paper chromatography is very useful in separating various constituents of coloured solutes present in a mixture of lime, ink, dyes etc.
➢ Separation of the 'Dyes' in Black Ink by Paper Chromatography
- The different coloured dyes present in black ink can be separated by performing paper chromatography.
 Layer Chromatography and Column Chromatography
Layer Chromatography and Column Chromatography
- Method:
(i) Take a thin and long strip of filter paper. Draw a pencil line on it, about 3 centimetres from one end.
(ii) Put a small drop of black ink on the filter paper strip at the centre of the pencil line. Let the ink dry.
(iii) When the drop of ink has dried, the filter paper strip is lowered into a tall glass jar containing water in its lower part (keeping the pencil line at the bottom).
(iv) The filter paper strip is held vertically by attacking its upper end to a glass rod with cello-tape (the glass rod being kept over the mouth of the glass jar). The lower end of the paper strip should dip in the water but the pencil line should remains above the water level in the jar.
- Observation: The water gradually rises up the filter paper strip by capillary action. As water moves up on the paper strip, it takes along the dyes present in ink. The dye which is more soluble in water dissolves first rises faster and produces a coloured spot on the paper at a higher position. The less soluble dyes dissolve a little later, rise slower and form coloured spots at lower heights. In this way, all the dyes present in black ink get separated.
 View Answer
View AnswerNote:
When the water reaches near the top end of the filter paper strip, the paper strip is removed from the jar and dried. The paper with its separate coloured spots is called a chromatogram.
- Result: The chromatogram obtained by using black ink in this experiment has three coloured spots on it. This means that the given sample of black ink has three different dyes mixed in it.
- Applications:
(i) To separate colours in a dye.
(ii) Pigments from natural colours.
(iii) Drugs from the blood.
6. Separation of a Mixture of Two Miscible Liquids
- Those liquids which mix together in all proportions and form a single layer are called miscible liquids. Alcohol and water are miscible liquids because they mix together in all proportions and form a single layer on mixing.
- To separate a mixture of two or more miscible liquids for which the difference in boiling points is less than 25 K, a fractional distillation process is used.
- A simple fractionating column is a long vertical glass tube filled with glass beads. The glass beads provide a large surface area for hot vapours to cool and condense respectively.
➢ Separate a mixture of Ethyl Alcohol and Water
- Ethyl alcohol and water are miscible liquids. The boiling point of ethyl alcohol is 78°C and the boiling point of water is 100°C, a mixture of ethyl alcohol and water can be separated by fractional distillation.
- Method: The mixture of ethyl alcohol and water is heated in a distillation flask fitted with a fractionating column. When the mixture is heated, both ethyl alcohol and water form vapours as their boiling points approach. The ethyl alcohol vapour and water vapour rise up in the fractionating column.
 Fractional DistillationThe upper part of the fractionating column is cooler, so as the hot vapours rise up in the column, they get cooled, condense and trickle back into the distillation flask. As the experiment goes on, the fractionating column warms up by the heat released by the condensed vapours.
Fractional DistillationThe upper part of the fractionating column is cooler, so as the hot vapours rise up in the column, they get cooled, condense and trickle back into the distillation flask. As the experiment goes on, the fractionating column warms up by the heat released by the condensed vapours.
After some time, the temperature at the top of the column is much less than at its bottom. When the temperature at the top of the fractionating column reaches 78°C, then ethyl alcohol vapour passes into the condenser, gets cooled and collects in a beaker kept at the end of the condenser.
The ethyl alcohol-water mixture is kept boiling at such a rate that the thermometer shows the boiling points of ethyl alcohol (78°C). In this way, all the ethyl alcohol distils over and gets separated.
7. Separation of the Gases of the Air
- Air is a mixture of gases like nitrogen, oxygen, argon, carbon dioxide, helium, neon etc. The major component of air is nitrogen (78.03%). The second major component of air is oxygen (20.99%) and the third major component of air is argon (0.93%).
- All these gases are obtained from the air on a large scale. This is because air is the cheapest source of these gases.
- Air is a homogeneous mixture and can be separated into its components by fractional distillation of liquid air.
➢ Separation of Components of Air
- The air is first filtered to remove dust, then water vapour and carbon dioxide are removed. If water vapour and carbon dioxide are not removed, they would become solid in the cooling process and block the pipes.
- Air is compressed to a high pressure and then cooled. This cooled air is then allowed to expand quickly into a chamber through a jet. This expansion cools the air even more.
- The process of compression, cooling and rapid expansion of air is repeated again and again makes the air cooler and cooler. Ultimately the air gets so cooled that it turns into a liquid. In this way, liquid air is obtained.
 Separation of Components of Air
Separation of Components of Air - The liquid air is fed into a tall fractional distillation column from near its bottom and warmed up slowly.
- Liquid nitrogen which is present in air, has the lowest boiling point, of, -196°C. So, on warming, liquid nitrogen boils off first to form nitrogen gas.
- Liquid argon which is present in the liquid air has a slightly higher boiling point of, -186°C, so liquid argon boils off next and collected as argon gas in the middle part of the fractional distillation column.
- Liquid oxygen also present in the liquid air has a still higher boiling point of, -183°C. so, liquid oxygen boils off last and collected as oxygen gas from the bottom of the fractional distillation column.
 Separation of Components of Air
Separation of Components of Air
8. Separation of Pure Copper Sulphate from an Impure Sample
The process involved in obtaining pure copper sulphate from its impure sample is crystallisation.
➢ Crystallisation
- The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation. Crystallisation is a process that separates a pure solid in the form of its crystals from a solution.
- Methods
(i) We take about 10 grams of impure copper sulphate and dissolve it in a minimum amount of water in a china dish to make copper sulphate solution.
(ii) Filter the copper sulphate solution to remove insoluble impurities.
(iii) Heat the copper sulphate solution gently on a water bath to evaporate water and obtain a saturated solution. This can be tested by dipping a glass rod in hot solution from time to time. When small crystals form on the glass rod, the solution is saturated. Then stop heating. Crystallization of Copper Sulphate(iv) Allow the hot, saturated solution of copper sulphate to cool slowly.
Crystallization of Copper Sulphate(iv) Allow the hot, saturated solution of copper sulphate to cool slowly.
(v) Crystals of pure copper sulphates are formed, impurities remain behind in the solution.
(vi) Separate the copper sulphate crystals from solution by filtration and dry. - Crystallisation is a better technique than evaporation to dryness because of the following reasons:
(i) Some solids decompose or get charred on heating to dryness during evaporation
(ii) The soluble impurities do not get removed in the process of evaporation. But such impurities get removed in crystallisation. - Application:
(i) Purification of salt that we get from seawater
(ii) Separation of crystals of alum (phitkari) from impure samples.
9. Supply of Drinking Water in a City
- In cities, drinking water is supplied from waterworks.
- In waterworks, the methods like sedimentation, decantation, loading, filtration and chlorination etc are used to remove undesirable materials from water.
- The source of water supply in a city is either a nearby river or a lake called reservoir.
- The river water and lake water usually contain suspended solid substances and some germs, so, before this water can be supplied to homes, it must be purified to remove suspended impurities as well as germs.
➢ Water purification system in waterworks
The purification of river water or lake water is done in the following steps:
- Sedimentation: The water is allowed to stand in big tanks, where heavier suspended impurities settle down. To increase the rate of sedimentation, alum is added to it. The impurities settle at the bottom.
- Filtration: The semi-clear water is allowed to pass through beds of sand, charcoal and gravel to remove suspended impurities.
- Removal of harmful organism or sterilization: The harmful bacteria in filtered water can cause very serious diseases such as typhoid, cholera etc. Thus, to the filtered water bleaching powder or chlorine gas is added. This kills the microorganisms and hence the water becomes fit for drinking. This water is directly pumped into overhead tanks for supply to a city.
 Process of Water Purification in Water Works
Process of Water Purification in Water Works
Physical and Chemical Changes
- There are some changes during which no new substances are formed. On the other hand, there are some other changes during which new substances are formed.
- So, on the basis of whether new substances are formed or not, we can classify all the changes into two groups: Physical changes and chemical changes.
 Physical and Chemical Changes
Physical and Chemical Changes
1. Physical Changes
- Those changes in which no new substances are formed are called physical changes.
- In a physical change, the substances involved do not change their identity. They can be easily returned to their original form by some physical process.
- This means that physical changes can be easily reversed to form the original substance.
- The changes in physical state, size and shape of substances are called physical changes.
- Examples:
(i) When ice is heated it melts to form water. Though ice and water look different, they are both made of water molecules. Thus no new chemical substance is formed during the melting of ice. So, the melting of ice to form water is a physical change.
(ii) When water is cooled, then water solidifies to form ice. This is called freezing of water. The freezing of water to form ice is also a physical change.
(iii) Some other examples of physical changes are Boiling of water, condensation of steam, the ringing of an electric bell and breaking of a glass.
 Physical Change i.e. Melting of Ice2. Chemical Changes
Physical Change i.e. Melting of Ice2. Chemical Changes
- Those changes in which new substances are formed are called chemical changes. A chemical change is also called a chemical reaction.
- In a chemical change, the substances involved, change their identity.
- They get converted into entirely new substances. The new substances usually cannot be returned to their original form.
- This means that chemical changes are usually irreversible.
- Example:
(i) When a magnesium wire is heated it burns in air to form a white powder called 'magnesium oxide'. This magnesium oxide is an entirely new substance. Thus a new chemical substance is formed during the burning of a magnesium wire is a chemical change.
(ii) Some other examples of chemical changes are: Burning of a candle, Burning of character, and burning of hydrogen in oxygen to form water.
 Burning of Magnesium
Burning of Magnesium
3. Differences between Physical and Chemical Changes
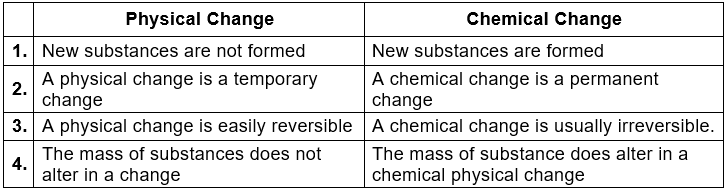
Pure Substance
- Pure substances are substances that are made up of only one kind of particles and have a fixed or constant structure.
- Pure substances are further classified as:
(i) Element: Substance that consists of only one type or kind of atom. An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means. Elements are mostly metals, non-metals or metalloids.
(ii) Compounds: when two or more elements are combined chemically in a fixed ratio. However, these substances can be broken down into separate elements by chemical methods. - Examples of Pure Substances: All elements are mostly pure substances. A few of them include gold, copper, oxygen, chlorine, diamond, etc. Compounds such as water, salt or crystals, baking soda amongst others are also grouped as pure substances.
➢ Characteristics and Properties Of Pure Substances
- Pure substances are mostly homogeneous in nature containing only one type of atoms or molecules.
- These substances mainly have a constant or uniform composition throughout.
- The substances have fixed boiling and melting points.
- A pure substance usually participates in a chemical reaction to form predictable products.
|
87 videos|369 docs|67 tests
|
FAQs on Separating Components of a Mixture, Physical and Chemical Changes & Pure Substances - Science Class 9
| 1. What is a mixture? |  |
| 2. What is the difference between a physical change and a chemical change? |  |
| 3. How can components of a mixture be separated? |  |
| 4. What is a pure substance? |  |
| 5. What are some applications of separating mixtures? |  |

|
Explore Courses for Class 9 exam
|

|
























