Points to Remember: Aliphatic Compounds | Organic Chemistry PDF Download
| Table of contents |

|
| Alkanes |

|
| Alkene and Alkynes |

|
| Halo-alkane |

|
| Ether |

|
| Grignard Reagent |

|
| Alcohols |

|
| Carboxylic Acid |

|
| Cyanide |

|
| Isocyanides |

|
Alkanes
A. Preparation:
➤ Sabatier-Senderens Reaction: When unsaturated hydrocarbons are treated with hydrogen in presence of metal catalyst (Ni/Pt) then saturated hydrocarbon is formed. It occurs by addition pathways. ➤ By Alkyl Halides: When alkyl halides are treated with Zn/AcOH, Zn-Cu/EtOH then saturated hydrocarbons are formed.
➤ By Alkyl Halides: When alkyl halides are treated with Zn/AcOH, Zn-Cu/EtOH then saturated hydrocarbons are formed. ➤ From Carboxylic Acid: When sodium salt of carboxylic acids are heated with soda lime (NaOH + CaO) then alkanes are formed.
➤ From Carboxylic Acid: When sodium salt of carboxylic acids are heated with soda lime (NaOH + CaO) then alkanes are formed. ➤ Wurtz Reaction: When ethereal solution of alkyl halides (preferably bromides/iodides) with small pieces of sodium metals then alkanes are formed. The reaction mechanism follows radical as well as ionic pathways. This is mostly useful for the preparation of even no. of carbon containing alkane. For odd no. of carbon containing alkane this will lead to the formation of mixture of products.
➤ Wurtz Reaction: When ethereal solution of alkyl halides (preferably bromides/iodides) with small pieces of sodium metals then alkanes are formed. The reaction mechanism follows radical as well as ionic pathways. This is mostly useful for the preparation of even no. of carbon containing alkane. For odd no. of carbon containing alkane this will lead to the formation of mixture of products.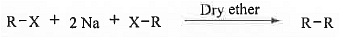
➤ Corey-House Synthesis: This is useful for the preparation of both even and odd no. of carbon containing alkane. The reaction follows ionic SN2 pathway. When lithium di-alkyl cuprate is treated with alkyl halide then alkane is formed.
R2CuLi + R;X.→ RR'
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of alkane depends on the intermolecular force of the alkane which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will b the intermolecular weight of the compound and hence, higher will be the bp/mp of the alkane. That's why bp/mp increase from methane to ethane to propane to butane and so on.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. As alkanes are non-polar, these are insoluble in polar solvent like water but become soluble in non-polar solvent like ether.
C. Reactions of Alkanes:
➤ Halogenation: Alkanes are very inert due to absence of pi-electrons. In presence of light these will react with halogen to produce alkyl halide via radical pathway.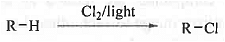
➤ Nitration: When gaseous nitric acid is treated with alkane at an elevated temperature then nitroalkane is formed.
➤ Sulphonation: When alkane is heated with fuming sulphuric acid then alkyl sulfonic .acid is formed.
➤ Isomerization: When straight chain alkanes are heated with strong Lewis acid like BF3 are found to convert to branched alkanes.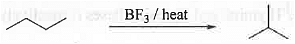
Alkene and Alkynes
A. Preparation:
➤ Dehydration of Alcohol: When alcohol is heated at 170°C in presence of strong acid like H2SO4, then alkene is formed
The ease of dehydration of alcohol are : 3° > 2° > 1°. This is due to that the reaction is passing through the formation of carbocationic intermediate and the order of stability of carbocalions are : 3° > 2° > 1°.
➤ Dehydrohalogenation of Alkyl Halide: When alkyl halide is dissolved in ethanolic K.OH and heated then alkene will form.
Here, also the order of the dehydrohalogenation of alkyl halide is : 3° > 2° > 1°. The reaction mechanism follows the E2 pathway.
➤ Dehalogenation of Vicinal Dihalides: When vicinal dihalides are dissolved into methanol and heated in presence of Zn, alkene is formed through dehalogenation.
➤ Heating Quaternary Ammonium Hydroxide: When quaternary ammonium hydroxide is heated then alkene is formed.
➤ Partial Reduction of Alkyne: When alkyne is treated with Na with liq. NH3, trans-alkene is formed while alkyne on reaction with hydrogen in presence of Lindler's catalyst produces cis-alkene.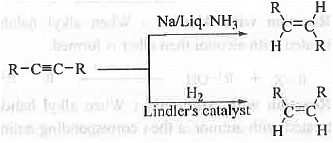
➤ Pyrolysis of Ester, Xanthate and N-oxide of Tertiary Amine: When ester/xanthate/N-oxide of tertiary amine is heated then alkene is formed.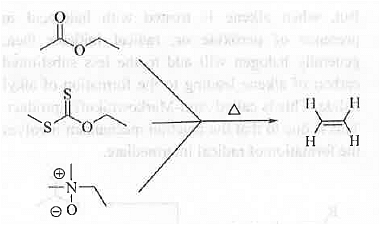
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of alkene depends on the intermolecular force of the alkene which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will b the intermolecular weight of the compound and hence, higher will be the bp/mp of the alkene. That's why bp/mp increase from ethene to propene to butene to pentene and so on.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. As alkenes are non-polar, these are insoluble in polar solvent like water but become soluble in non-polar solvent like ether.
C. Reactions of Alkenes:
➤ Addition of Hydrogen: When alkene is treated with hydrogen in presence of catalyst like Pt or, Pd or, Raney Ni at room temperature then alkane is formed.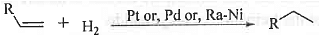
➤ Addition of Halogen: When alkene is treated with halogens by dissolving it into CCI4 at room temperature, vicinal di-halide is formed.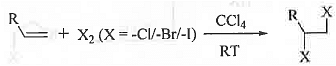
➤ Addition of Halo-acid: When halo-acid is treated with alkene, generally halogen will add to the more substituted carbon of alkene leading to the formation of alkyl halide. This is called Markownikoffs product. This is due to that the reaction mechanism involves the formation carbocationic intermediate.
But, when alkene is treated with halo-acid in presence of peroxide or, radical initiator then, generally halogen will add to the less substituted carbon of alkene leading to the formation of alkyl halide. This is called Anti-Markownikoffs product. This is due to that the reaction mechanism involves the formation of radical intermediate.
Halo-alkane
A. Preparation:
➤ From Alkane: When alkane is treated with chlorine in presence of UV-light then alkyl halide is formed.
➤ Hunsdiecker Reaction: When silver salt of aliphatic carboxylic acid is treated with bromine in carbon tetrachloride medium then alkyl bromide is formed.
➤ By Addition of HX: When halo-acid is treated with alkene, generally halogen will add to the more substituted carbon of alkene leading to the formation of alkyl halide.
But, when alkene is treated with halo-acid in presence of peroxide then, generally halogen will add to the less substituted carbon of alkene leading to the formation of alkyl halide.
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of alkyl halide depends on the intermolecular force of the alkyl halide which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will b the intermolecular weight of the compound and hence,
higher will be the bp/mp of the alkene. That's why bp/mp increase from methyl halide to ethyl halide and so on. Due to the similar reason bp/mp increase from methyl chloride to methyl iodide.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. As alkyl halides are non-polar, these are insoluble in polar solvent like water but become soluble in non-polar solvent like ether.
C. Reactions of Alkyl Halides:
Reaction with NaCN and AgCN: When alkyl halide is treated with NaCN then alkyl cyanide is formed while alkyl halide on reaction with AgCN produces alkyl isocyanide.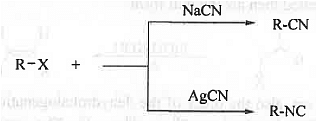
➤ Wurtz Reaction: When ethereal solution of alkyl halides (preferably bromides/iodides) with small pieces of sodium metals then alkanes are formed. The reaction mechanism follows radical as well as ionic pathways. This is mostly useful for the preparation of even no. of carbon containing alkane. For odd no. of carbon containing alkane this will lead to the formation of mixture of products.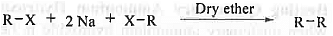
➤ Hydrolysis: When alkyl halide is treated with aq. KOH then substitution reaction will occur resulting in the formation of alcohol while on treatment with ale. KOH gives alkene via elimination pathway.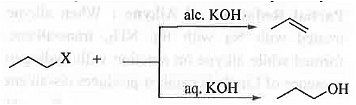
➤ Reaction with Alcohol: When alkyl halide is treated with alcohol then ether is formed.
➤ Reaction with Ammonia: When alkyl halide is treated with ammonia then corresponding amine is formed.
Ether
A.Preparation:
➤ From Isobutylene: When isobutylene is treated with acid and then the resulting tert-butyl cation is reacted with alcohol then ether is formed. it is only useful for the preparation of symmetrical ether.
➤ From Isobutylene: When isobutylene is treated with acid and then the resulting tert-butyl cation is reacted with alcohol then ether is formed. It is highly useful for the tert-butyl group containing ether.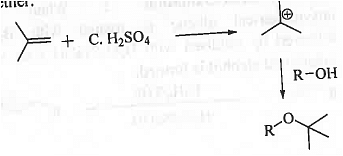
➤ By Using Diazomethane: When ether is treated with di azomethane in presence of Al(OEt)3 then ether is formed. It is highly useful for the preparation of methyl group containing ether.
➤ Williamson's Synthesis: When alkyl halide is treated with sodium alkoxide then ether is formed. It is susceptible towards steric crowding. It is useful for the preparation of both symmetrical and unsymmetrical ethers.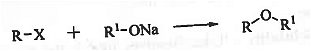
B. Physical Properties
➤ Boiling Point (B.P.) and Melting Point (M.P.) : B.P. and M.P. of ethers depends on the intermolecular force of the ethers which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will b the intermolecular weight of the compound and hence, higher will be the bp/mp of the ether. That's why bp/mp increase from dimethyl ether to ethyl methyl ether and so on.
➤ Solubility : 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. Ether contains hydrophilic oxygen containing part which is responsible for its solubility in water through the formation of inter molecular H bond with water.
molecule and non polar hydrophobic part which is responsible for insolubilty of ether in water.that ' s why 3C containing ether is fully soluble in water while the higher members are insoluble in water.
C.Reactions:
➤ Reaction with HI: When ether is treated with I mole H°I then alkyl iodide and alcohol are formed. here the reaction will pass through the Sn2 pathway. hence, he iodide ion will add to that carbon part which is less sterically hindered. but if one of the alkyl part is tert butyl then the reaction will follow Sn1 pathway. hence, the iodide ion will add to the tert butyl group. but if ether is treated with excess of HI then only alkyl iodides are formed.
Grignard Reagent
A. Preparation:
➤ Alkyl Halide: When alkyl halide (cholride/ bromide) is treated with mangesium halide is formed.
B. Reaction:
➤ Preparation of alkane: When Grignard reagent reacts with compounds having active hydrogen atom (such as alcohol, active methylene group containing compounds like EAA then alkane is formed.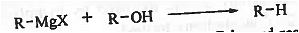
➤ Preparation of alcohols: When Grignard reagent is treated with HCHO/any other aldehyde/ any ketone followed by hydrolysis then 1°/2°/3° alcohols are formed. 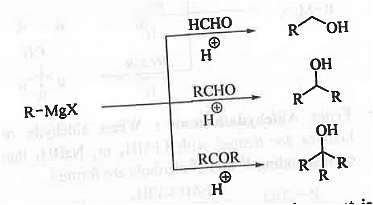
➤ Preparation of acid: When Grignard reagent is reacted with carbon dioxide and then acidic work is done then carboxylic acid is formed.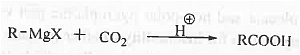
➤ Preparation of Aldehyde: When Grignard reagent is treated with ethyl formate then aldehyde is formed.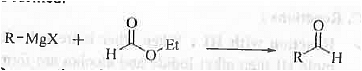
➤ Preparation of ketone: When Grignard reagent is treated with ethyl ketone ; When Grignard reagent is treated with ethyl acetate and its higher analogues then ketone is formed.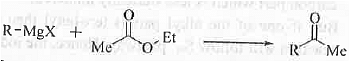
➤ Preparation of Ester: When Grignard reagent is treated with ethyl chloro formate then eater is formed.
➤ Preparation of Amine: When Grignard reagent is treated with chloramine then amine is formed.
➤ Preparation of cyanide: : When Grignard reagent is treated with cyanogen bromide then alkyl cynide is formed.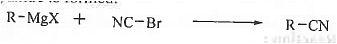
Alcohols
A. preparation:
➤ By Using Grignard Reagent: When Grignard reagent is treated with HCHO/any other aldehyde/any ketone followed by hydrolysis then 1°/2°/3° alcohols are formed.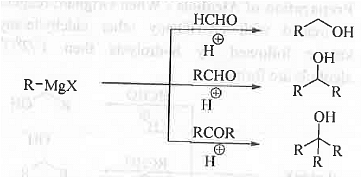
➤ From Aldehyde/ ketone: : When aldehyde or, ketones are treated with LiAlH4 or, NaBH4 then corresponding 1° and 2° alcohols are formed.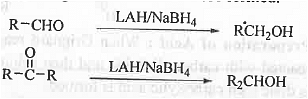
➤ From Carboxylic acid: When carboxylic acid is reduced with strong reducing agent like LAH then primary alcohol is formed.
➤ Oxymercuration-Demercuration Reaction: When an unsymmetrical alkene is treated with Hg(OAc)2 followed by reduction with NaBH4 then more substituted alcohol is formed.
➤ Hydrobo ration-Oxidation: When an unsymmetrical alkene is treated with B2H6 follow ed by oxidised with H2O2/ NaOH then less substituted alcohol is formed.
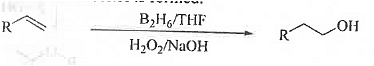
B. Physical Properties :
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of alcohol depends on the intermolecular force o f alcohols which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, high er will be the intermolecular weight of the compound and hence, higher will be the bp/mp of the alcohol. That's why bp/inp increase from methanol to ethanol and so on.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. Alcohol contains hydrophilic -OH part which is responsible for its solubility in water through the formation of inter-molecular H-bond with water molecule and non-polar hydrophobic part which is responsible for in solubility of ether in water. That's why 4C containing alcohol is fully soluble in water while the higher members are insoluble in water.
C. Reactions :
➤ Reaction with Grignard Reagent: When alcohol is treated with Grignard Reagent then alkane is formed.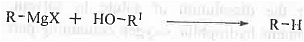
➤ Reaction with Carboxylic Acid: When alcohol is treated with carboxylic acid in acidic medium then ester is formed.
R-COOH + HO-R1 → R-COOR1
➤ Reaction with SOCI2/PCI3/PCI5/POCI3: When alcohol is reacted with chlorinating agent SOCI2/PCI3/PCI5/POCI3 then alkyl chloride is formed.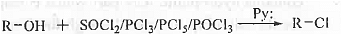
➤ Oxidation of Alcohol: When alcohol is oxidised with mild oxidising agent P.C.C or, P.D.C then aldehyde/ketone is formed. But, on oxidation with stronger oxidising agent like K2Cr2O7 in presence of H2SO4 will produce corresponding carboxylic acid.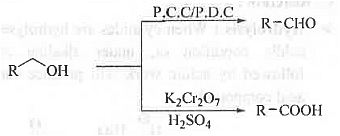
Carboxylic Acid
A. Preparation:
➤ From Alcohol: When primary alcohol is oxidised with stronger oxidising agent like K2Cr20 7 in presence of H2S04 corresponding carboxylic acid is formed.
➤ From Tri-chloro alkane: When tri-chloro alkane is hydrolysed then carboxylic acid is formed.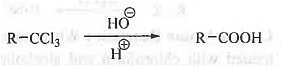
➤ From Ketomethyl Group Containing Substance: When 2-hydroxypropane is treated with Cl2/NaOH and then acidic work up then is done then acetic acid is formed.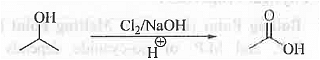
➤ From Benzaldehyde: When acetaldehyde is treated with K2Cr2O7 and H2SO4 then benzoic acid is formed.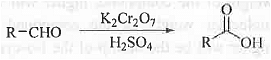
➤ From Cyanide Compounds: When cyanides are hydrolysed under acidic condition or, under alkaline condition followed by acidic work will produce carboxylic acid compound.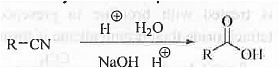
➤ By Hydrolysis of Ester/Amide/Anhydride/Acyl Chloride: Ester/amide/anhydride/acyl chloride class of compounds on hydrolysis under alkaline condition and followed by acidic work up gives the corresponding carboxylic acids in cases.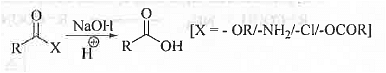
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of carboxylic acid depends on the intermolecular force of carboxylic acid which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will b the intermolecular weight of the compound and hence, higher will be the bp/mp of the carboxylic acid. That's why bp/mp increase from formic acid to acetic acid and so on.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. Carboxylic acid contains hydrophilic -COOH part which is responsible for its solubility in water through the formation of inter-molecular H-bond with water molecule and non-polar hydrophobic part which is responsible for insolubility of ether in water. That's why 4C containing carboxylic acid is fully soluble in water while the higher members are insoluble in water.
C. Reactions:
➤ Reaction with Diazomethanc: When acetic acid is treated with diazomethane then methyl acetate is formed.
➤ Reduction: When carboxylic acid is treated with Lithium aluminium hydride then corresponding alcohol is formed.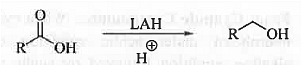
➤ Hunsdiecker Reaction: When silver carboxylate is treated with bromine in presence of carbon tetrachloride then bromo alkane is formed.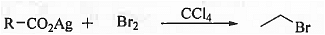
➤ Reaction with Ammonia: When carboxylic acid is treated with ammonia then ammonium carboxylate is formed which on heating produces corresponding amide which on treatment with P2O5 will produce corresponding cyanide compound.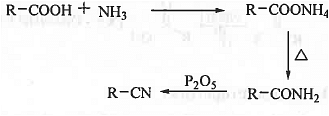
Cyanide
A. Preparation:
➤ From Carboxylic Acid: When carboxylic acid is treated with ammonia then ammonium carboxylate is formed which on heating produces corresponding amide which on treatment with P2O5 will produce corresponding cyanide compound.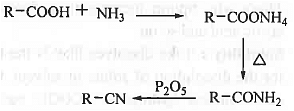
➤ From Alkyl Halide: When alkyl halide is treated with NaCN then alkyl cyanide is formed.
➤ From Grignard Reagent: When Grignard reagent is treated with cyanogen bromide then alkyl cyanide is formed.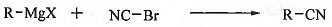
➤ From Oxime: When oxime is treated with dehydrating agent like P205 then cyanide compound is formed.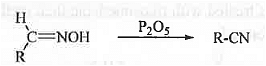
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.) : B.P. and M.P. of cyanide depends on the intermolecular force of cyanide which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will be the intermolecular weight of the compound and hence, higher will be the bp/mp of the cyanide. That's why bp/mp increase from HCN to CH3CN and so on.
➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. Alcohol contains hydrophilic -CN part which is responsible for its solubility in water through the formation of inter-molecular H-bond with water molecule and non-polar hydrophobic part which is responsible for insolubility of ether in water. That's why lower member cyanides are soluble in water while higher members are insoluble in water.
C. Reaction:
➤ Hydrolysis: When cyanides are hydrolysed under acidic condition or, under alkaline condition followed by acidic work will produce carboxylic acid compound.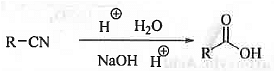
➤ Reduction: When cyanides are reduced with LAH then corresponding primary amine is formed.
Isocyanides
A. Preparation:
➤ From Alkyl Halide: When alkyl halide is treated with AgCN then alkyl isocyanide.
➤ Carbyl Amine Reaction: When primary amine is treated with chloroform and alcoholic KOH then isocyanide is formed.
R - NH2 + CHC13 + ale. KOH → R-NC
B. Physical Properties:
➤ Boiling Point (B.P.) and Melting Point (M.P.): B.P. and M.P. of iso-cyanide depends on the intermolecular force of iso-cyanide which in turn depends on the effective molecular weight of the compound. Greater is the effective molecular weight of the compound, higher will be the intermolecular weight of the compound and hence, higher will be the bp/mp of the iso-cyanide. That's why bp/mp increase from CH3NC to C2H5NC and so on. ➤ Solubility: 'Like dissolves like' is the thumb rule for the dissolution of solute in solvent. As isocyanide does not able to form inter-molecular H- bond with water molecule, iso-cyanides are insoluble in water.
C. Reaction:
➤ Hydrolysis: When iso-cyanides are hydrolysed under acidic condition then amine is formed.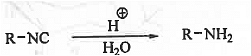
➤ Reduction: When iso-cyanides are reduced with LAH then corresponding secondary amine is formed.
|
35 videos|92 docs|46 tests
|
FAQs on Points to Remember: Aliphatic Compounds - Organic Chemistry
| 1. What are alkanes and how do they differ from alkenes and alkynes? |  |
| 2. What are halo-alkanes and how are they different from other aliphatic compounds? |  |
| 3. What is the significance of Grignard reagents in aliphatic chemistry? |  |
| 4. How do alcohols differ from carboxylic acids in terms of their chemical structure and properties? |  |
| 5. What are cyanides and isocyanides, and how do they differ in terms of their chemical structure and reactivity? |  |

|
Explore Courses for Chemistry exam
|

|

















