Points to Remember: Organometallic Chemistry & Bioinorganic Chemistry | Inorganic Chemistry PDF Download
Points to be Remembered
1. EAN Rule: It is 18 e- rule (sometimes referred as 36 e- rule also). It indicates the stability of a complex. If a complex contains 18 e- then it will neither undergo oxidation nor undergo reduction. But, if a complex contains 17 e- i.e. less than 18 e- then it will undergo reduction to attain more stable 18 e- configuration. But, if a complex contains 19 e- i.e. more than 18 e- then it will undergo oxidation to attain more stable 18 e' configuration.
Example - Ni(CO)4 has {10 + 4(2)} = 18 e-. Hence, it is highly stable.
But, in case of V(CO)6 the no. of electrons present are {5 + 6(2)} = 17. Hence, it will undergo reduction to produce more stable V(CO)6 ion.
2. Wade's Rule : Determination of Borane Type: Wade's rule is useful for the determination of the type of borane. It consist of the following steps:
- First count the total no. of electrons present in the species.
- Then count the total no. of polyhedron electrons present in the species.
- Then determine the no. of vertices (n) present in the complex.
- Finally, if [TEC - PEC]/2 value equals to (n+1), (n+2) and (n+3) then the borane will be closo, nido and arachno type.
3. π-Acidic Ligand: The ligand which donates electrons to the metal through o-donation pathway and accept electrons from metal through jt-back acceptance pathway is called π-acidic ligand.
Example - CO is a π-acid ligand. Its mechanism is as follows:
From the M.O. (Molecular Orbital) of CO, it is evident that the HOMO and LUMO of CO are filled σab and vacant π* orbitals. During the formation of metal carbonyls, CO will donate electrons from filled σ- orbital to the metal vacant p or, d orbital. Due to such electron donation by CO metal become electron rich. Hence, the complex become unstable. To remove this instability, metal will donate excess electrons from its filled d-orbital to CO vacant n orbital. This σ-electron donation and π-electrons acceptance by CO occurs simultaneously. This simultaneous electron donation and acceptance is called 'Synergistic Effect'. The orbital overlaps in metal carbonyls is as follows: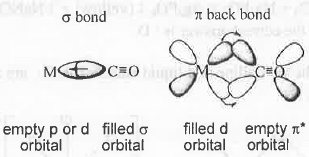 4. Fisher Carbene vs. Schrock Carbene: Fisher carbene is electrophilic in nature while Schrock carbene is nucleophilic in nature. In case of Fisher carbene rc-acidic ligands like CO etc. are attached with the carbene carbon while in case of Schrock carbene non-ru-acidic ligands like -Me etc. are attached with the carbene carbon. Hence, in case of Fisher carbene synergistic effect is observed. Hence, the metal present in Fisher carbene should be at low oxidation state to facilitate the synergistic effect while the metal present in Schrock carbene should be at high oxidation state to facilitate the electron donation process by non-π-acidic ligands.
4. Fisher Carbene vs. Schrock Carbene: Fisher carbene is electrophilic in nature while Schrock carbene is nucleophilic in nature. In case of Fisher carbene rc-acidic ligands like CO etc. are attached with the carbene carbon while in case of Schrock carbene non-ru-acidic ligands like -Me etc. are attached with the carbene carbon. Hence, in case of Fisher carbene synergistic effect is observed. Hence, the metal present in Fisher carbene should be at low oxidation state to facilitate the synergistic effect while the metal present in Schrock carbene should be at high oxidation state to facilitate the electron donation process by non-π-acidic ligands.
5. Conditions for Allowed Molecular/Electronic Transition: For an electronic transition to become allowed, the following conditioned has to be satisfied:
- Change in spin-multiplicity will be zero i.e. ΔS = 0.
- Change in orbital multiplicity will be zero and
 i.e. ΔA = 0,
i.e. ΔA = 0, [A for Σ, II and Δ are 0, 1 and 2 respectively].
[A for Σ, II and Δ are 0, 1 and 2 respectively]. - Nuclear charge should not be changed i.e. + —> + and - —> - transitions are allowed.
On considering these three basic conditions for an allowed molecular transition, the allowed transition would be : JΣu+ --> JΣg+ and lπg --> lΣu+.
6. Hydroformylation: Hydroformylation reaction is a reaction where an alkene converted to an aldehyde with one carbon more preferably at the terminal position. The involving steps are:
H2 + CO2(CO)8 ⇋ 2HCo(CO)4
HCo(CO)4 ⇋ HCo(CO)3 + CO Ligand dissociation
CH3CH = CH2 + HCo(CO)3 ⇋ CH3CH2CH2Co(CO)3 Intermediate
CH3CH2CH2Co(CO)3+ CO ⇋ CH3CH2CH2COCo(CO)3 Acyl intermediate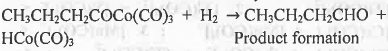
7. Carbonic Anhydrase: It is an Zn containing enzyme which catalyse the interconversion of CO2 and Carbonates through the hydration of CO2 and dehydration of H2CO3. Zn(II) is present at the active site of the enzyme.
H2O + CO2 ⇋ H2CO3 ; H2CO3 ⇋ H2O + CO2
8. Carboxypeptidase A: It is an enzyme which hydrolyses the peptide bonds of C-terminal residue. Here, also Zn(II) ion is present at the active site of the enzyme.
9. Hemocyanin: Cu is present in Hemocyanin. The main biological function of Hemocyanin is the transport of oxygen throughout the bodies of some invertebrate animals.
10. Hemoglobin: Fe (II) is present at the active site of the metallo- protein, Hemoglobin. The main function of hemoglobin is that it transports oxygen from lungs to the tissues.
11. Cytochrome b: Fe (II) is present at the active site of Cytochrome b. It acts as the part of electron transport chain.
12. Vitamin B12: Vitamin B12 contains Co at its active site. It takes part in group transfer reactions.
13. Nitrogenase: It is an which involved in nitrogen fixation. At the active site of nitrogenase, Fe and Mo are present.
14. Myoglobin: Fe(II) is present at the active site of myoglobin. Its main function is to store oxygen.
|
50 videos|92 docs|41 tests
|





















