Class 9 Science Chapter 3 Practice Question Answers - Atoms and Molecules
Multiple Choice Questions
Q1: A sample of pure water, irrespective of its source contains 11.1% hydrogen and 88.9% oxygen. The data supports
(a) law of constant proportions
(b) law of conservation of mass
(c) law of reciprocal proportions
(d) law of multiple proportions
Ans: (a)
Water obtained from any source contains hydrogen and oxygen in the same proportion by mass. Hence, the data supports the law of constant proportions.
Q2: Identify the correct statements.
1. In a compound such as water, the ratio of the mass of hydrogen to the mass of oxygen is always 8 : 1.
2. If 9 g of water is decomposed, 1 g of hydrogen and 8 g of oxygen are always obtained.
3. In ammonia, nitrogen and hydrogen are always present in the ratio 3 : 14 by mass.
4. Many compounds are composed of two or more elements and each such compound has the same elements in the same proportions.
(a) 1 and 3
(b) 1, 2 and 3
(c) 2 and 4
(d) All of these
Ans: (c)
In a compound such as water, the ratio of the mass of hydrogen to the mass of oxygen is always 1 : 8. In ammonia, nitrogen and hydrogen are always present in the ratio 14 : 3 by mass.
Q3: The atomic mass of calcium (Ca) is 40 g. The number of moles in 60 g of calcium are
(a) 0.5 mol
(b) 2.0 mol
(c) 1.5 mol
(d) 0.75 mol
Ans: (c)
Q4: What mass of carbon-di-oxide (CO2) will contain 3.011 × 1023 molecules?
(a) 11.0 g
(b) 22.0 g
(c) 4.4 g
(d) 44.0 g
Ans: (b)
6.022 × 1023 molecules of CO2 corresponds to 44 g 3.011 × 1023 molecules of CO2 corresponds to = 22 g
Q5: The valency of nitrogen in ammonia (NH3) is
(a) 2
(b) 0
(c) 3
(d) 4
Ans: (c)
Q6: The molecular formula P2O5 means that
(a) a molecule contains 2 atoms of P and 5 atoms of O
(b) the ratio of the mass of P to the mass of O in the molecule is 2 : 5
(c) there are twice as many P atoms in the molecule as there are O atoms
(d) the ratio of the mass of P to the mass of O in the molecule is 5 : 2.
Ans : (a)
Molecular formula represents the actual number of atoms of different elements present in one molecule of the compound.
Q7: Which of the following represents a polyatomic ion?
(a) Sulphide
(b) Chloride
(c) Sulphate
(d) Nitride
Ans : (c)
Sulphate  ion consists of group of atoms. Sulphate is a polyatomic ion.
ion consists of group of atoms. Sulphate is a polyatomic ion.
Q8: How many elements are present in one formula unit of Al(OH)3?
(a) 3
(b) 4
(c) 5
(d) 6
Ans: (a)
One formula unit of Al(OH)3 contains aluminium, oxygen and hydrogen.
Q9: The formula of chloride of a metal M is MCl3, then the formula of the phosphate of metal M will be (a) MPO4
(b) M2PO4
(c) M3PO4
(d) M2(PO4)3
Ans: (a)
Q10: Which of the following is a triatomic molecule?
(a) Carbon-di-oxide
(b) Ammonia
(c) Helium
(d) Sugar
Ans: (a)
Carbon-di-oxide contains one atom of carbon and two atoms of oxygen.
Q11: All samples of carbon-di-oxide contain carbon and oxygen in the mass ratio 3 : 8. This is in agreement with the law of
(a) conservation of mass
(b) constant proportions
(c) multiple proportions
(d) gaseous volumes
Ans: (b)
Law of constant proportions states that a chemical compound is always made up of the same elements combined together in the same fixed proportion by mass.
Q12: How many grams of H2SO4 are present in 0.25 mole of H2SO4 ?
(a) 2.45
(b) 24.5
(c) 0.245
(d) 0.25
Ans: (b)
Mass = Number of moles × Molar mass
= 0 2. 5 × 98 = 24. g
Q13: Select the incorrect match.
1. N2O4 -Dinitrogen tetroxide
2. HCl-Hydrogen chloride
3. CO-Carbon dioxide
4. PCl5 -Phosphorus trichloride
(a) 1 and 2
(b) 3 and 4
(c) 1 and 3
(d) 2 and 4
Ans: (b)
CO - Carbon monoxide.
PCl5 - Phosphorus pentachloride.
Q14: Atomicity of sulphur is
(a) 8
(b) 4
(c) 2
(d) 1
Ans : (a)
Sulphur exists as S8 molecule
Q15: Valency of silver in Ag2S is
(a) 1
(b) 2
(c) 0
(d) 3
Ans: (a) Fill in the blanks.
Fill in the blanks.
Q16: In ionic compounds, the charge on each ion is used to determine the ......... of the compound.
Ans: chemical formula
In ionic compounds, the charge on each ion is used to determine the 'chemical formula' of the compound. This means that the positive and negative charges of the ions should balance out to form a neutral compound. The number of positive and negative ions in the compound is used to create the chemical formula.
Q17: The Avogadro constant ......... is defined as the number of atoms in exactly ......... of carbon-12.
Ans: 6.022 × 1023 , 12 g
The 'Avogadro constant', also known as Avogadro's number, is '6.022 × 1023'. This is defined as the number of atoms in exactly '12 g' of carbon-12. This number helps in measuring very small particles like atoms and molecules which cannot be counted individually.
Q18: The abbreviation used for lengthy names of elements are termed as their .........
Ans: symbol.
The 'abbreviation' used for lengthy names of elements are termed as their 'symbol'. This is done for convenience and ease of use in writing chemical equations. For example, the symbol for Hydrogen is H, for Oxygen it is O, and for Carbon it is C.
Q19: Mole is link between the .......... and .........
Ans: mass of atoms & number of atoms.
'Mole' is a link between the 'mass of atoms' and 'number of atoms'. This concept is crucial in chemistry as it helps in understanding the relationship between the mass of a substance and the number of particles it contains. A mole of any substance contains the same number of particles, which is approximately 6.022 × 1023.
Q20: The valency of an ion is ......... to the charge on the ion.
Ans: equal.
The 'valency' of an ion is 'equal' to the charge on the ion. Valency is a measure of the ability of an atom to bond with other atoms. If an ion has a positive charge, its valency is equal to the number of electrons it has lost. If an ion has a negative charge, its valency is equal to the number of electrons it has gained.
True/False
Q21: In a pure chemical compound, elements are always present in a definite proportion by mass.
Ans: True
The statement that in a pure chemical compound, elements are always present in a definite proportion by mass is true. This is known as the law of definite proportions, which states that a chemical compound always contains exactly the same proportion of elements by mass, regardless of the amount of substance.
Q22: Mass of 1 mole of a substance is called its formula mass.
Ans: False
The statement that the mass of 1 mole of a substance is called its formula mass is false. The mass of 1 mole of a substance is called its molar mass, not formula mass. Molar mass is the mass of one mole of a substance and it is expressed in grams per mole (g/mol). This concept is essential in chemistry for calculating quantities in chemical reactions.
Q23: Water is an atom.
Ans: False
The statement that water is an atom is false. Water is not an atom but a molecule. Water, or H2O, consists of two hydrogen atoms and one oxygen atom. These atoms are chemically bonded together to form a water molecule. This is a basic concept in chemistry, distinguishing between atoms (the smallest units of elements) and molecules (combinations of two or more atoms).
Q24: Formula mass of Na2O is 62 amu.
Ans: True
The formula mass of Na2O is indeed 62 amu. This is calculated by adding the atomic masses of its constituent atoms as per the periodic table: sodium (Na) is 23 amu and oxygen (O) is 16 amu. Since there are two atoms of sodium, the total mass becomes 2*23 + 16 = 62 amu.
Q25: Those particles which have more or less electrons than the normal atoms are called ions.
Ans: True
The statement that particles which have more or less electrons than the normal atoms are called ions is true. Atoms become ions by gaining or losing electrons. If an atom gains electrons, it becomes a negatively charged ion or anion. If an atom loses electrons, it becomes a positively charged ion or cation.
Matching Questions
Direction: In the section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct.
Q26:  Ans: (a) P - 4, Q - 3, R - 2, S - 1
Ans: (a) P - 4, Q - 3, R - 2, S - 1
Q27:
 Ans: (b) P - 2, Q - 1, R - 4, S - 3
Ans: (b) P - 2, Q - 1, R - 4, S - 3
Q28: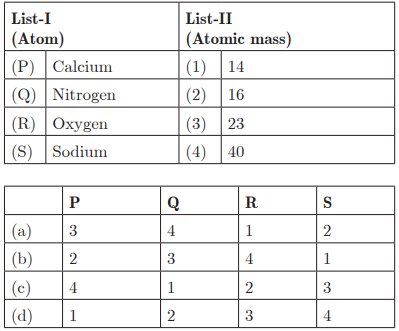 Ans: (c) P - 4, Q - 1, R - 2, S - 3
Ans: (c) P - 4, Q - 1, R - 2, S - 3
Q29:
Ans : (c) P - 2, Q - 1, R - 4, S - 3
Q30: Ans : (d) P - 2, Q - 1, R - 4, S - 3
Ans : (d) P - 2, Q - 1, R - 4, S - 3
|
88 videos|369 docs|67 tests
|

|
Explore Courses for Class 9 exam
|

|

















