Principle of Calorimetry | Chemistry for EmSAT Achieve PDF Download
The cosmos consists of both substance and energy. Substance comprises atoms and molecules, while energy drives their constant movement, manifesting as either vibrational oscillations or collisions. This motion generates thermal energy, commonly referred to as heat, which permeates all forms of matter, even the most frigid reaches of space. This piece explores the technique used to quantify heat exchange within chemical reactions or other physical phenomena, termed calorimetry.
What is Calorimetry?
Calorimetry is the practice or discipline of gauging alterations in a body's state variables to determine the heat exchange linked to shifts in its conditions, such as alterations in physical state or phase transitions within certain parameters. It involves the utilization of a calorimeter to carry out measurements.
Calorimeter Principle
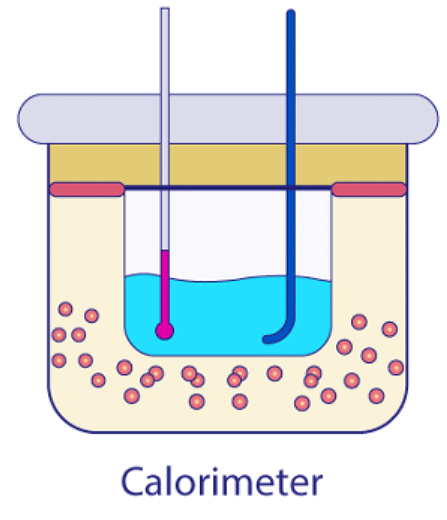
When two objects with varying temperatures, ideally one solid and one liquid, are brought into contact, heat flows from the warmer object to the cooler one until they reach thermal equilibrium. The warmer object releases heat while the cooler one absorbs it. This process aligns with the principle of calorimetry, which embodies the law of conservation of energy, stating that the total heat relinquished by the warmer object equals the total heat acquired by the cooler object.
Heat Lost = Heat Gained
The heat transfer in a system is calculated using the formula,
q=mcΔt
Where
- q is the measure of heat transfer
- m is the mass of the body
- c is the specific heat of the body
- Δt is the change in the temperature
|
195 videos|213 docs|157 tests
|
FAQs on Principle of Calorimetry - Chemistry for EmSAT Achieve
| 1. What is the principle of calorimetry? |  |
| 2. What are the types of calorimetry? |  |
| 3. How is calorimetry used in real-life applications? |  |
| 4. What are some common calorimeter problems encountered in experiments? |  |
| 5. How does understanding heat transfer and calorimetry help in studying thermal energy? |  |

|
Explore Courses for EmSAT Achieve exam
|

|
















