Year 11 Exam > Year 11 Notes > Physics for GCSE/IGCSE > Radioactive Decay
Radioactive Decay | Physics for GCSE/IGCSE - Year 11 PDF Download
| Table of contents |

|
| Effect of Nuclear Size on Decay |

|
| Change to a New Element |

|
| Reducing Neutron Number |

|
| Decay Equations |

|
Effect of Nuclear Size on Decay
- The nuclei that are most stable typically possess a similar number of protons and neutrons. If there is an excess of protons, the repulsive force generated by their like positive charges can lead to the nucleus repelling when it grows large.
- Consequently, nuclei with an imbalance of protons or neutrons are more prone to decay into smaller nuclei until they reach a stable form with a balanced number of protons and neutrons.
- Isotopes of an element may exhibit radioactivity due to various reasons such as an excess of protons or neutrons within the nucleus or the nucleus being overly heavy. For instance, the isotope of hydrogen-1 exemplifies this phenomenon.

- Hydrogen-1 (H-1) represents the stable nucleus of hydrogen.
- Deuterium (H-2) contains an additional neutron.
- Tritium (H-3) carries an extra neutron, resulting in a neutron-to-proton ratio of 2:1, rendering it significantly more unstable than H-1 or H-2.
- Nuclei become too heavy when they possess an excess of protons and neutrons, weakening the forces that bind them together and potentially leading to decay.
- Uranium-238, employed in nuclear fission, exemplifies this phenomenon, boasting 238 protons and neutrons.
- Uranium-238 undergoes decay, gradually reducing its mass number as it transforms into another element, achieved through alpha (α) or beta (β) decay processes.

Change to a New Element
- In α-decay or β-decay processes, the nucleus undergoes a transformation into a different element.
- The original nucleus is commonly referred to as the parent nucleus.
- Conversely, the nucleus of the resulting element is termed the daughter nucleus.
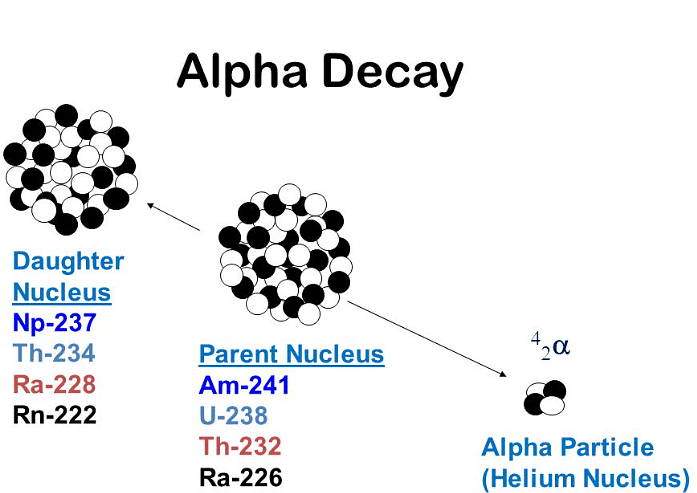
- The daughter nucleus constitutes a distinct element due to its varying proton and/or nucleon count compared to the original parent nucleus.
- This distinction is evident when observing a graph plotting neutron number (N) against proton number (Z).
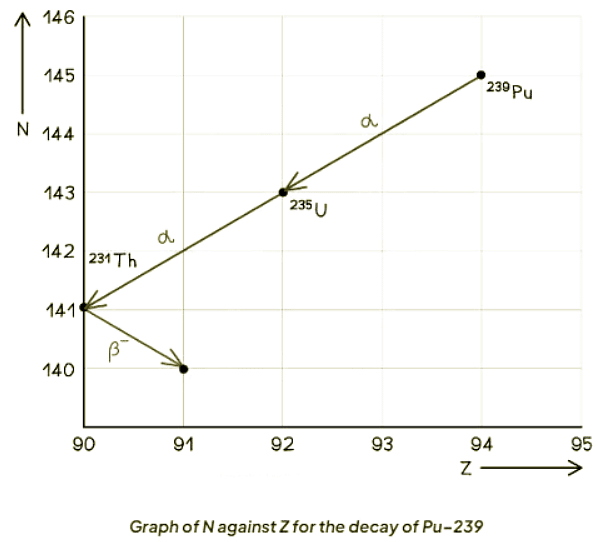
- When Pu-239 undergoes alpha decay to become U-235, it sheds 2 protons and 2 neutrons.
- Uranium (U) and Plutonium (Pu) are entirely distinct elements from each other.
Question for Radioactive DecayTry yourself: What is the effect of an excess of protons or neutrons on the stability of a nucleus?View Solution
Reducing Neutron Number
- A nucleus undergoes decay to enhance its stability by diminishing the surplus of neutrons, achieved through alpha or beta decay mechanisms.
- Excess energy within the nucleus is released in the form of radiation, frequently in the form of gamma radiation.
Alpha Decay
- In alpha decay, an unstable nucleus emits an alpha particle.
- This process results in the formation of an entirely new element.

- An alpha particle comprises a helium nucleus, consisting of 2 protons and 2 neutrons.
- Upon emission from the unstable nucleus, the mass number and atomic number of the nucleus undergo alteration.
- The mass number diminishes by 4, while the atomic number decreases by 2.
- Additionally, the charge on the nucleus decreases by 2, attributed to the +1 charge carried by each proton.
Beta Decay
- In beta decay, a neutron converts into a proton and an electron.
- The emitted electron exits the nucleus, while the proton remains within.
- This process results in the formation of a new element due to the alteration in the atomic number.
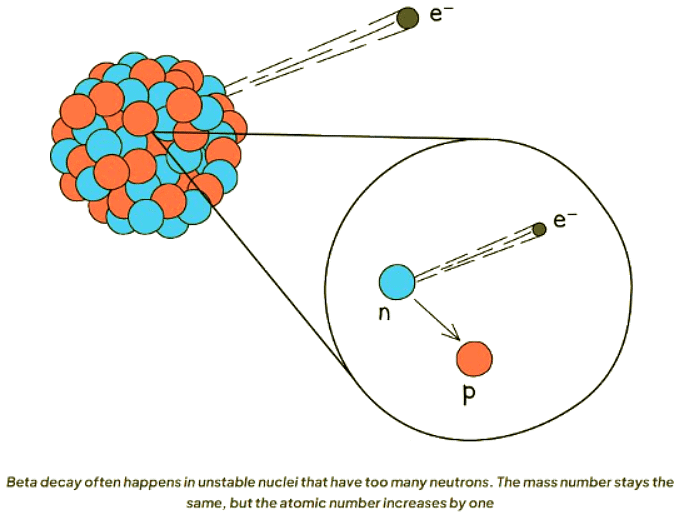
- A beta particle, characterized by a high-speed electron, possesses a mass number of 0.
- This is because the electron's mass is insignificantly small compared to that of neutrons and protons, thus maintaining the mass number of the decaying nucleus.
- With an atomic number of -1, electrons cause an increase of 1 in the atomic number of the new nucleus to uphold the overall atomic number before and after decay.
- In beta decay, as exemplified by carbon-14 transitioning to nitrogen-14, the process entails the emission of a beta particle, represented as an electron in the equation.
Gamma Decay
- In gamma decay, an unstable nucleus emits a gamma ray.
- This process reduces the nucleus's energy without altering its structure.

- The emitted gamma ray possesses considerable energy but lacks both mass and charge.
Decay Equations
- Radioactive decay events can be represented using a decay equation.
- A decay equation resembles a chemical reaction equation.
- The particles existing prior to decay are depicted before the arrow.
- The particles generated during decay are shown after the arrow.
- In decay equations, the total mass and atomic numbers before the reaction must equal those after the reaction.
- The decay equation for Polonium-212 undergoing alpha decay shows it transforming into Lead-208 and emitting an alpha particle.
- An alpha particle can also be expressed as a helium nucleus, denoted by the symbol He.
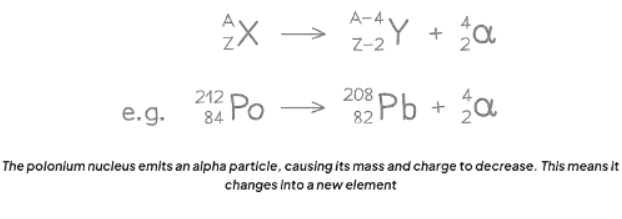
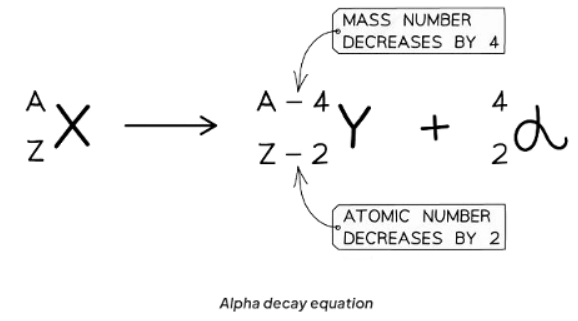
Alpha Decay Equation
When an alpha particle is discharged from the unstable nucleus, alterations occur in its mass number and atomic number.
- The mass number diminishes by 4.
- The atomic number diminishes by 2.
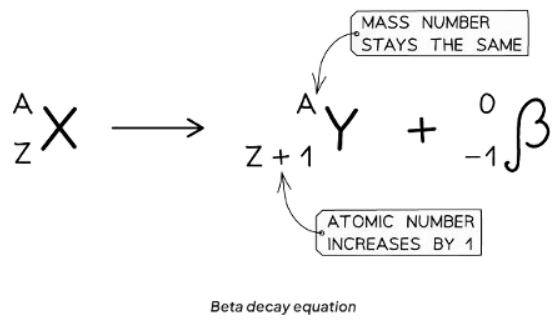
Beta Decay Equation
In beta decay, a neutron transforms into a proton and an electron.
- The emitted electron is known as a beta particle.
- The proton remains within the nucleus.
Gamma Decay
- Gamma rays emitted during decay possess substantial energy but lack mass or charge.
- An example of gamma decay involves Uranium-238.
- It's noteworthy that the mass number and atomic number of the unstable nucleus remain constant throughout the decay process.

The document Radioactive Decay | Physics for GCSE/IGCSE - Year 11 is a part of the Year 11 Course Physics for GCSE/IGCSE.
All you need of Year 11 at this link: Year 11
|
127 videos|148 docs|35 tests
|
FAQs on Radioactive Decay - Physics for GCSE/IGCSE - Year 11
| 1. What is the effect of nuclear size on radioactive decay? |  |
Ans. The larger the nucleus, the more unstable it is, leading to a higher likelihood of radioactive decay. This is because larger nuclei have a greater ratio of protons to neutrons, causing instability.
| 2. How does a too heavy nucleus result in decay? |  |
Ans. A nucleus that is too heavy may decay in order to achieve a more stable configuration. This can involve the emission of alpha or beta particles, as well as gamma radiation.
| 3. What is the uranium-238 decay chain? |  |
Ans. The uranium-238 decay chain is a series of radioactive decays that uranium-238 undergoes to eventually reach a stable configuration. This chain includes the emission of alpha and beta particles.
| 4. How does a change in mass number lead to a new element? |  |
Ans. When an unstable nucleus undergoes radioactive decay, it may lose or gain protons, leading to a change in mass number. If the change results in a different element, a new element is formed.
| 5. Can you explain the processes of alpha and beta decay in nuclear physics? |  |
Ans. Alpha decay involves the emission of an alpha particle, which consists of 2 protons and 2 neutrons. Beta decay, on the other hand, involves the emission of either an electron (beta-minus decay) or a positron (beta-plus decay) from the nucleus.

|
Explore Courses for Year 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches

















