Raman Spectroscopy | Physical Chemistry PDF Download
Raman Scattering
Raman scattering produces scattered photons with a different frequency depending on the source and the vibrational and rotational properties of the scattered molecules. Raman spectroscopy works on the principle of Raman scattering. It is used to study materials by chemists and physicists. In the olden days, to record spectra, a mercury lamp and photographic plates were used; in modern days, lasers are used. Sir CV Raman was awarded the Nobel Prize for Physics in the year 1930. C V Raman, along with his student K S Krishnan, discovered Raman’s scattering.
What is Raman Scattering?
Raman scattering is defined as the scattering of photons by excited molecules at higher energy levels. It is also known as the Raman effect. The photons are inelastically scattered, which means that the kinetic energy of an incident particle is either lost or increased and is composed of Stokes and anti-Stokes portions.
Inelastic scattering of photons is similar to the concept of an inelastic collision, which states that the total microscopic kinetic energy is not conserved. In an elastic collision, the transfer of kinetic energy occurs, but the scattering will still be inelastic like in Compton scattering.
similar to Rayleigh scattering, Raman scattering also depends on the polarizability of the molecules. The intensity of Rayleigh scattering is about 10−3 to 10−4 compared to the intensity of the exciting source. The photon’s energy and the state of the molecule after the scattering events are unchanged. In Raman scattering, the frequency of photons presents in the monochromatic light changes when interacted with the vibrational states, or modes, of a molecule.
LASER, considered intense monochromatic light in Raman scattering, can give rise to scattered light, which contains one or more sidebands offset by rotational or vibrational energy differences. The sidebands produced include frequencies containing information about the scattering medium, and hence they are used in remote sensing.
Degrees of Freedom
The degree of freedom (DOF) is defined as a number of independent parameters that determine the configuration of the physical system. Following is the degrees of freedom formula:
DF = n − 1
where DF is the degree of freedom and n is the number of samples given.
For Raman scattering, 3 N is the DOF (for any given chemical compound), where N is the number of atoms in the compound 3 N is DOF because each atom moves in x, y, and z-direction ie; they possess translational, rotational, and vibrational motion.
What Is Raman Spectroscopy?
C.V. Raman discovered Raman spectroscopy in 1928 to study the vibrational, rotational, and low-frequency modes of the molecules. It finds application mainly in chemistry to get the information related to fingerprints.
Principle of Raman Spectroscopy
The principle behind Raman spectroscopy is that the monochromatic radiation is passed through the sample such that the radiation may get reflected, absorbed, or scattered. The scattered photons have a different frequency from the incident photon as the vibration and rotational property vary. This results in the change of wavelength,, which is studied in the IR spectra.
The difference between the incident photon and the scattered photon is known as the Raman shift. When the energy associated with the scattered photons is less than the energy of an incident photon, the scattering is known as Stokes scattering. When the energy of the scattered photons is more than the incident photon, the scattering is known as anti-Stokes scattering.
Types of Raman Spectroscopy
Following are the types:
- Resonance Raman Spectroscopy (RRS)
- Surface-enhanced Raman Spectroscopy (SERS)
- Micro-Raman Spectroscopy
- Non-linear Raman Spectroscopic Techniques
What Is Raman Spectrometer?
Raman spectrometer is an instrument that consists of one or more single-coloured light sources and lenses and filters to focus the light and to differentiate the reflected and scattered light, respectively. A prism is used for splitting the light into its components which has a detector to detect the weak light. Later the spectrum is obtained on the monitor to analyze the information.
What Is Raman Spectra?
To analyse the Raman effect, the wavelength of the scattered photon is converted to wavenumber. These wavenumbers are plotted on the x-y plane. The wavenumbers are taken along the x-axis and the Raman intensity is taken on the y-axis. The difference between the wavenumbers and the intensity is known as the Raman spectrum.
Applications of Raman Effect
- Raman amplification: this is based on the Raman scattering where the lower frequency photons are pumped to a high-frequency regime with a surplus amount of energy. This method is applicable to telecommunications.
- Supercontinuum generation: In optics, supercontinuum is formed using the Raman spectra, which results in smooth spectra as the initial spectra are built spontaneously which is later amplified to higher energy.
- Raman spectroscopy works on the basis of Raman effect and finds applications in various fields like in nanotechnology to understand the structure of nanowires, in biology and medicine where the low-frequency DNAs and proteins are studied and chemistry to understand the structure of molecules and their bonds.
- Raman scattering is used in remote sensing and planetary exploration.
- Raman scattering is used to sense the minerals in Mars.
What is Spectroscopy?
Spectroscopy means the dispersion of light into component colours. In simple words, it is a method to measure how much light is absorbed by a chemical substance and at what intensity of light passes through it. As per analytical science, every element or compound has unique characteristic spectrum. Each compound absorbs and disperses light over a certain range of wavelengths.
Type of interaction between light and material
In spectroscopy, the type of interaction between light and the material is usually -:
- Absorption spectroscopy
- Emission spectroscopy
- Elastic scattering
- reflection spectroscopy
- Impedance spectroscopy
- Inelastic scattering
- Coherent or resonance spectroscopy
In Chemistry, Spectroscopy helps to study or analyse various chemical compounds or elements, whereas, in Physics, it helps to determine the makeup of the atmospheres of planets.
Types of Spectroscopy
- Acoustic resonance
- Time-resolved
- Photoemission
- X-ray photoelectron
- Circular Dichroism
- IR Spectroscopy (Infrared spectroscopy)
- Raman spectroscopy
Infrared Spectroscopy
The type of spectroscopy which deals with the infrared region of the electromagnetic spectrum is Infrared Spectroscopy. The rays of the infrared region have longer wavelength whereas having a lower frequency than light. Infrared spectroscopy is based on absorption spectroscopy.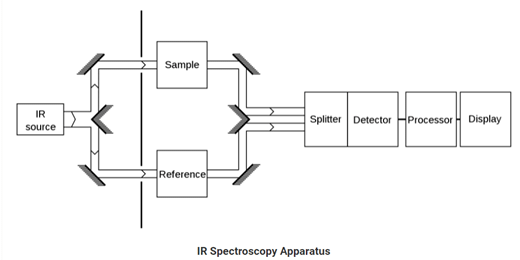
Raman Spectroscopy
Raman Spectroscopy is a spectroscopic technique which is used to analyze vibrational, rotational, and other low-frequency modes in a system. Raman’s spectroscopy is commonly used in the branch of chemistry to provide a fingerprint by which molecules can be identified. As the name suggests, this phenomenon is named after Sir C. V. Raman. This phenomenon relies on inelastic scattering of monochromatic light which is also known as Raman scattering. The energy of the laser photons shifts up & down due to the interaction of the light with the molecules or phonons of an object. This up down shift of laser photon forms the vibrational modes of an object or system.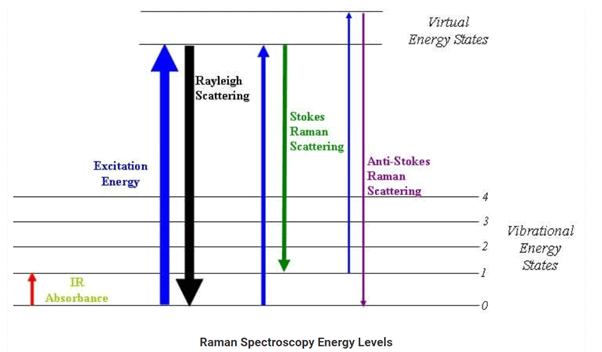
Other advanced types of Raman spectroscopy are surface-enhanced Raman, resonance Raman, tip-enhanced Raman, polarized Raman, stimulated Raman (analogous to stimulated emission), transmission Raman, spatially offset Raman, and hyper Raman.
|
84 videos|142 docs|67 tests
|
FAQs on Raman Spectroscopy - Physical Chemistry
| 1. What is Raman scattering? |  |
| 2. How does Raman spectroscopy work? |  |
| 3. What are the applications of Raman spectroscopy? |  |
| 4. What are the advantages of Raman spectroscopy? |  |
| 5. What are the limitations of Raman spectroscopy? |  |





















