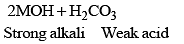Chemical & Physical Properties of Alkali Metals | Inorganic Chemistry PDF Download
Alkali metals belong to the s-block elements occupying the leftmost side of the periodic table.
- Alkali metals readily lose electrons, making them count among the most reactive elements on Earth.
- In general ‘alkali’ refers to the basic or alkaline nature of their metal hydroxides. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids.

- Alkali elements are Lithium(Li), Sodium(Na), Potassium (K), Rubidium (Ru), Cesium (Cs) and Francium (Fr) occupying successive periods from first to seven. Francium is a radioactive element with a very low half-life.
- The main reason why hydrogen (H) is not considered an alkali metal is that it is mostly found as a gas when the temperature and pressure are normal. Hydrogen can show properties or transform into an alkali metal when it is exposed to extremely high pressure.
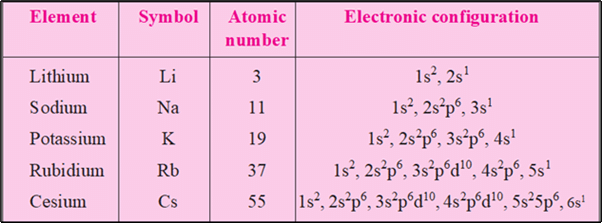
Electronic Configuration of Alkali Metals
- Alkali metals have one electron in their valence shell.
- The electronic configuration is given by ns1. For example, the electronic configuration of lithium is given by 1ns1 2ns1.
- They tend to lose the outer shell electron to form cations with charge +1 (monovalent ions).
- This makes them the most electropositive elements and due to the same reason, they are not found in the pure state.
Trends in Physical Properties of Alkali Metals
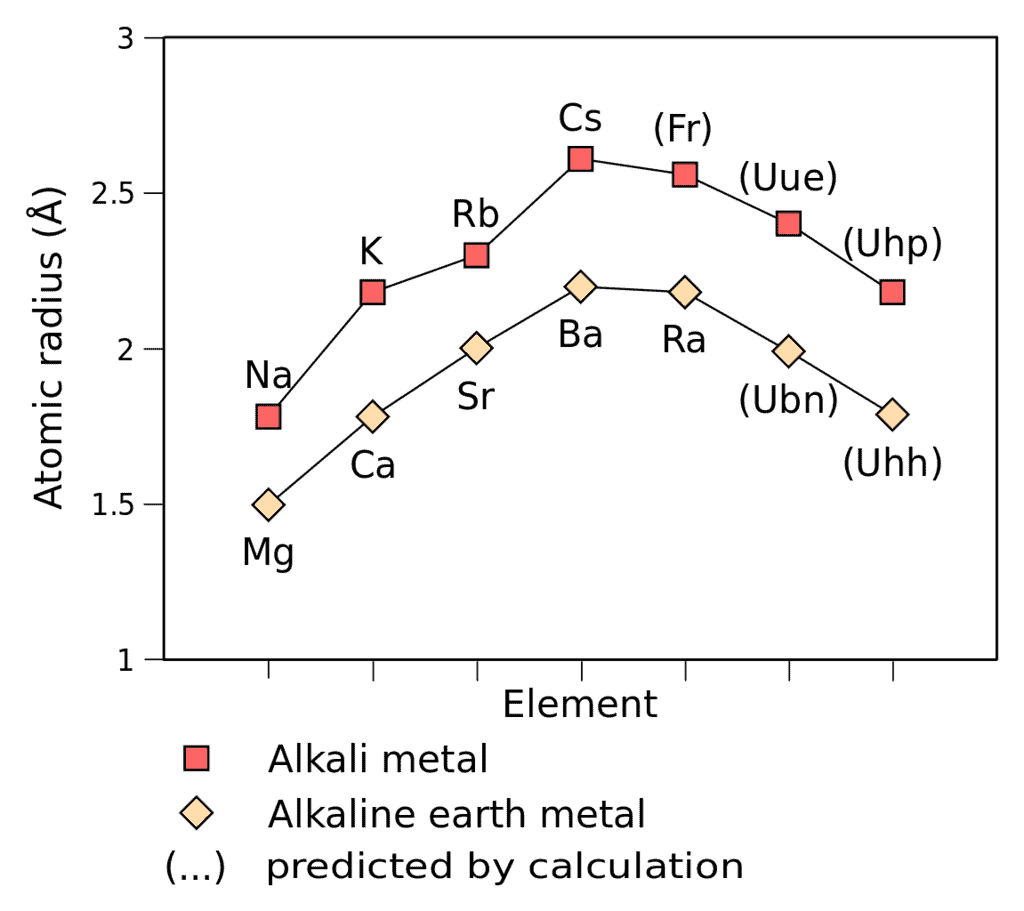
1. Atomic and Ionic Radii
- Atomic and ionic radii of elements increase, regularly down the column. Also, every alkali metal has the largest radii of any other element in the corresponding period.
- Alkali metals readily lose an electron and become cationic. The cationic radius is smaller than the neutral atom.
- The relative ionic radii also increase down the column.
Increasing order of Atomic and Ionic Radius: Li ˂ Na ˂ K ˂ Rb ˂ Cs and Li+ ˂ Na+ ˂ K+ ˂ Rb+ ˂ Cs+
2. Density
- All are light metals. The densities of metals Li, Na, and K Are lighter than water.
- Density gradually increases in moving down from Li to Cs.
- Potassium is, however, lighter than sodium.
- Sequence of densities: Li < Na < K < Rb < Cs
3. Structures of the Metals, Hardness & Cohesive Energy
- At normal temperatures, all the Group 1 metals adopt a body-centred cubic type of lattice with a coordination number of 8. However, at very low temperatures lithium forms a hexagonal close-packed structure with a coordination number of 12.
- The cohesive energy is the force holding the atoms or ions together in the solid.
- The atoms become larger on descending the group from lithium to caesium, so the bonds are weaker, the cohesive energy decreases, and the softness of the metals increases.
- The crystal structures of Li2O, Na2O, K2O, and Rb2O are anti-fluorite structures, and Cs2O has an anti-CdCl2 layer structure.
4. Melting and boiling points
- The cohesive energy decreases down the group, and the melting points decrease correspondingly.
- The energy binding the atoms in the crystal lattices of these metals is relatively low on account of a single electron in the valency shell.
- Consequently, the metals have low melting and boiling points. These decrease in moving down from Li to Cs as the metallic bond strength decreases or cohesive force decreases.
5. Ionisation Energies & Electropositive Character
- Due to their large size, the outermost electron is far from the nucleus and can easily be removed.
- Their ionization potentials are relatively low. Thus, the metals have a great tendency to lose the ns1 electron to change into M + ions.
- These metals are highly electropositive. As the ionization potential decreases from Li to Cs, the electropositive character increases, i.e. metallic character increases.
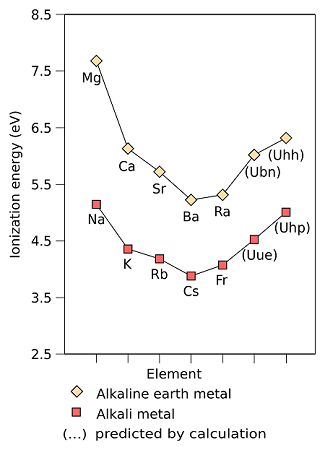
- The reactivity of these metals increases from Li to Cs.

- The ns1 electron is so loosely held that even the low-energy photons (light) can eject this electron from the metal surface. This property is termed a photoelectronic effect. K and Cs are used in photoelectric cells which are sensitive to blue light.
6. Oxidation States
The alkali metals can lose their ns1 electron quite easily to form a univalent positive ion, M+.
- The ion has a stable configuration of inert gas. These metals are univalent and show electrovalency, i.e., form electrovalent compounds.
- Since the electron configuration of M + ions is similar to those of inert gases, these ions have no unpaired electrons and consequently are colourless and diamagnetic.
7. Hydration of Ions, Hydrated Radii, and Hydration Energy
The salts of alkali metals are ionic and soluble in water. The solubility is due to the test that cations get hydrated by water molecules M+ + aq —→ [M(aq)]+
- The smaller the cation, the greater the degree of its hydration of M + ions & decrease from Li to Cs+.
- Consequently, the radii of the hydrated ion decrease from Li+ to Cs+
- The ionic conductance of these hydrated ions increases from [Li(aq)] + to [Cs(aq)]+.
- Hydration of ions is an exothermic process.
- The energy released when one gram mole of an ion is dissolved in water to get it hydrated is called hydration energy. Since the degree of hydration decreases from Li + to Cs+, the hydration energy of alkali metal ions also decreases from Li+ to Cs+.
- Some water molecules touch the metal ions and bond to them, forming a complex. These water molecules constitute the primary shell of water. Thus Li+ is tetrahedrally surrounded by four water molecules sp3 hybridization.
- With the heavier ions, particularly Rb+ and Cs+, the number of water molecules increases to six. VSEPR theory predicts an octahedral structure (d2sp3 hybridization).
- A secondary layer of water molecules further hydrates the ions, though these are only held by weak ion-dipole attractive forces.
8. Conductivity
The alkali metals are good conductors of heat and electricity. This is due to the presence of loosely held valency electrons which are free to move throughout the metal structure.
9. Heat of Atomization
- The heat of atomization decreases from Li to Cs.
- This is due to the decrease in the metallic bond strength from Li to Cs
10. Flame Colors & Spectra
- The alkali metals and their salts impart a characteristic colour to a flame.
- The colour arises from electronic transitions in short-lived species which are formed
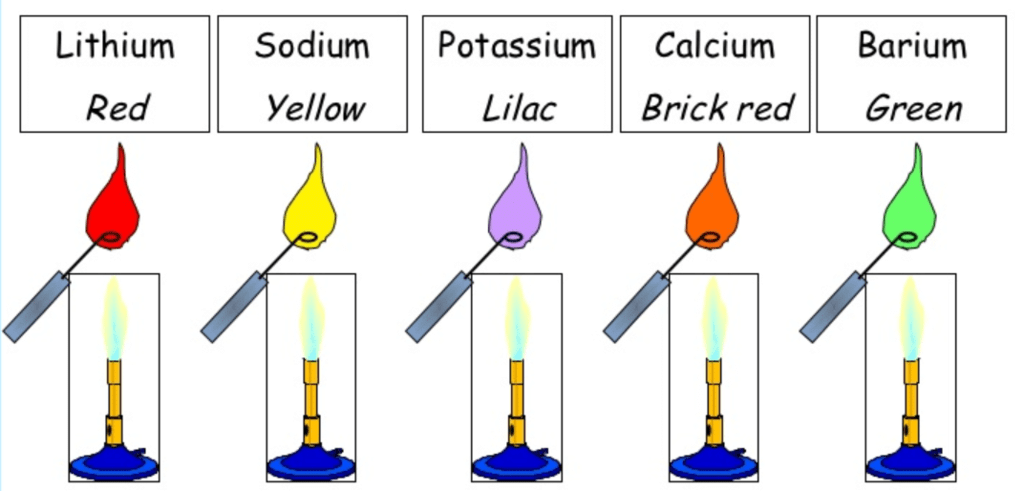
- The reason for flame coloration is that the energy of the flame causes an excitation of the outermost electrons which on return to their original position give out the energy so absorbed in the visible region.
- The energy released is minimal in the case of Li + and increases from Li + to Cs+. Thus the frequency of the light emitted increases by the formula E = hν. The frequency of light in lithium is minimum which corresponds to the red region of the spectra.
- Compounds of Group 1 metals are typically white, except those where the anion is coloured. For example sodium chromate Na2(CrO4) (yellow), potassium dichromate K2[Cr2O7] (orange), and potassium permanganate K[MnO4] (deep purple).
11. Reduction Potential
The substances that can donate electrons are reducing agents. Reducing ability is, related to the ease of electron donation or lower ionization energy.
As ionization energy decreases down the column, reducing property is expected to increase from Lithium to Cesium. While reducing ability increases from Sodium to Cesium, Lithium has the highest reduction potential (-3.04V) and is the strongest reducing agent of all elements.
Reduction potential and reducing ability depend on the combined energy difference of three processes:
- Sublimation of the atom,
- Ionization to the metal ion,
- Hydration of the ion with water.
Lithium, being the smallest ion, its hydration enthalpy is much higher than others and compensates more than its higher ionization enthalpy: ENa ˂ EK ˂ ERb ˂ ECs ˂ RLi
Chemical Properties of Alkali Metals
1. Action with Air
- On exposure to moist air, all alkali metals except lithium tarnish quickly. The effect of the atmosphere increases from Li to Cs. These are, therefore always kept under kerosene oil to protect them from the air.
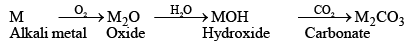
- Lithium, when heated in air, combines with nitrogen to form nitride, it is due to the diagonal relationship with magnesium.
2. Action with Water
- Alkali metals decompose water with the evolution of hydrogen gas.
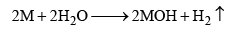
3. Action with Hydrogen
- The alkali metals combine directly with hydrogen to form crystalline hydrides of the formula M. H. These hydrides are ionic and contain the hydrides ion. H–
2M +H2 ——→ 2MH - The ionic character of the bonds in these hydrides increases from LiH to CsH and the stability decreases in the same order.
- They are powerful reducing agents, especially at high temperatures.
SiCl4 + 4NaH ——→ SiH4 + 4NaCl
4. Action with Oxygen (Oxides and Hydroxides)
- Down the group affinity towards oxygen increases.
- The metals all burn in the air to form oxides, though the product varies depending on the metal. lithium forms the monoxide Li2O (and some peroxide Li2O2), sodium forms the peroxide Na2O2 and some monoxide Na2O), and the others form superoxides of the type MO2.
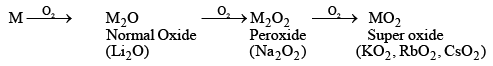
- The normal oxides ‘M2O’ react with water to form hydroxides.
M2O + H2O ——→ 2MOH - The basic nature of the oxides (M2O) increases gradually on moving down in the group. The hydroxides (MOH) are colourless, strong alkaline, and corrosive compounds.
- These are soluble in water and dissolve with the evolution of heat. The hydroxides are thermally stable except LiOH.
- The relative strength of the hydroxides increases from LiOH to CsOH.
CsOH > RbOH > KOH > NaOH > LiOH
5. Action with Halogens
- The alkali metals directly react with halogens forming the halides of the type MX. 2M + X2 → 2MX
- Except for certain lithium haloids, the metal halides are ionic compounds (M+X–).
- The halides are crystalline and have high melting and boiling points. The fused halides are good conductors of electricity and are used for the preparation of alkali metals.
- All halides except LiF dissolve in water.
- The alkali metal halides react with the halogens and interhalogen compounds forming ionic polyhalide compounds.
KI + I2 → K[I3], KBr + ICI → K[BrICI], KF + BrF3 → K[BrF4]
6. Nature of Oxysalts
- Alkali metals readily react with oxyacids forming corresponding salts with the evolution of hydrogen.
- Lithium salt behaves abnormally due to the polarizing power of Li+ ions (small size) and lattice energy effects.
7. Nature of Carbonates and Bicarbonates
- As the electropositive character increases from Li to Cs, the solubility of the carbonates increases in the same order
Cs2CO3 > Rb2CO3 > K2CO3 > Na2CO3 > Li2CO3 - Li2CO3 decomposes on heating and is insoluble in water. The aqueous solution of carbonates is alkaline. This is due to hydrolysis as carbonates are salts of strong bases and weak acid (H2CO3) carbonic acid).
 ⇆
⇆ 
- The bicarbonates, MHCO3, of the alkali metals, except lithium, are known in solid-state. The bicarbonates are soluble in water. On heating, bicarbonates decompose into carbonates with the evolution of CO2.
2MHCO3 ——→ +M2CO3 + H2O + CO2 - The abnormal behaviour of Li2CO3 towards heat can be explained in the following manner. The Li + ion exerts a strong polarizing action and distorts the electron cloud of the nearby atom the large CO32- ion.
- This results in the weakening of the C—O bond and the strengthening of the Li—O bond. This ultimately facilitates the decomposition of Li2CO3 into Li2O and CO2.
- The lattice energy of Li2O is higher than the lattice energy of carbonate. This also favours the decomposition of Li2CO3.
- Lithium due to its less electropositive nature, does not form solid bicarbonate and LiHCO 3 exists in solutions only.
8. Nature of nitrates
Nitrates of the type, MNO3, are known. These are colourless, soluble in water, and electrovalent in nature. The nitrates do not undergo hydrolysis. Except for LiNO3, the other nitrates decompose into nitrites and oxygen.
2MNO3 ——→ 2MNO2 + O2
Lithium nitrate decomposes to oxide on heating, it is due to the diagonal relationship with magnesium.
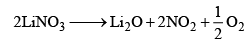
9. Nature of Sulphates
Sulphates of the type M2SO4 are known. Except for Li2SO4, other sulphates are soluble in water. The sulfates when fused with carbon form sulphides.
M2SO4 + 4C ——→ M2S + 4CO
The sulphates of alkali metals form double salts with the sulphates of the trivalent metals like Fe, Al, Cr, etc. The double sulphates crystallize with a large number of water molecules potash alum K2SO4, Al2(SO4)3.24H2O consists of 24 water molecules. Sulphate of lithium is not known to form alum.
10. Action of Liquid Ammonia
The alkali metals dissolve in liquid ammonia without the evolution of hydrogen. The colour of the dilute solutions is blue. The metal atom loses electrons and it combines with the ammonia molecule.
M → M+ (in liquid ammonia) + e (ammoniated)
M+(x + y) NH3 → [M(NH3)x]+ + e(NH3)y solvated electron
On heating its blue colour changes to bronze. It is an ammoniated electron that is responsible for colour.
The solutions are good conductors of electricity and have strong reducing properties. The solutions are paramagnetic.
When dry ammonia is passed over hot metal, amides are formed.
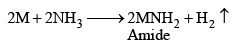
The amides are decomposed by cold water with the evolution of NH3 MNH2 + 2NH3 ——→ MOH + NH3
Recent studies proved the existence of Li(NH3)4, a golden yellow solid.
11. Formation of Alloys
The alkali metals form alloys amongst themselves and with other metals. These combine with mercury readily forming amalgams.
12. Complex Formation
Alkali metals have a very little tendency to form complexes, Lithium being small in size forms certain complexes but this tendency decreases as the size increases.
13. Diagonal Relationship: Similarities with Magnesium
Lithium shows a resemblance with magnesium, an element of group IIA. This resemblance is termed a diagonal relationship.
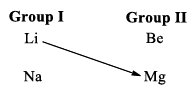
The reasons for the diagonal relationship are the following:
- Electronegativities of Li and Mg are quite comparable (Li = 1.00 Mg = 1.20)
- Atomic radii and ionic radii of Li and Mg are not very much different.
Atomic radii (Å) Li-1.23, Mg-1.36
Ionic radii (Å) Li+-00.60, Mg2+-0.65 - Both have high polarizing power (ionic potential) Polarizing power

- Cations with large ionic potentials tend to polarize the anions and give partial covalent character to compounds.
Lithium resembles magnesium in the following respects.
- Both Li and Mg are harder and have higher melting points than the other metals in their respective groups.
- Li like Mg decomposes water slowly to liberate hydrogen.
- 2Li + 2H2O ——→ 2LiOH + H2; Mg + 2H2O ——→ Mg(OH)2 + H2
- Both elements combine with nitrogen on heating.
- 6Li + N2 ——→ 2Li3N; 3Mg + N2 ——→ Mg3 + N2
- Both the nitrides are decomposed by water with the evolution of ammonia (NH3)
- Li3N + 3H2O ——→ 3LiOH + NH3; Mg3N2 + 6H2O ——→ 3Mg(OH)2 + 2NH3
- Both Li and Mg combine with carbon on heating
- 2Li + 3H2O ——→ 3Li2C2; Mg + 2C ——→ MgC2
- Both the carbides yield C2H2 with water.
- Lithium forms the only monoxide when heated in oxygen.
Mg also forms the monoxide - 4Li + O2 ——→ 2Li2O; 2Mg + O2 ——→ 2MgO
- Both are less soluble in water.
- Hydroxides of Li and Mg are weak bases and are slightly soluble in water. Both decompose on heating
- 2LiOH ——→ Li2O + H2O; Mg(OH)2 ——→ MgO + H2O
- Lithium fluoride, phosphate, oxalate, and carbonate like the corresponding salts of Mg, are sparingly soluble in water.
- Carbonates of Li and Mg decompose on heating.
- Li2CO3 ——→ Li2O + CO2; MgCO3 ——→ MgO + CO2
- Nitrates of Li and Mg decompose on heating giving a mixture of nitrogen dioxide and oxygen.
- 4Li2 NO3 ——→ 2Li2O + 4NO2 + O2 ; 2Mg(NO3)2 ——→ 2MgO + 4NO2 + O2
Compounds of Sodium
1. Sodium Oxide,
Na2O 2NaNO3 + 10 Na ——→ 6Na 2O + N2 ; 2NaNO2 + 6 Na ——→ 4Na 2O + N2
Properties
NO2O + H2O ——→ 2NaOH
2. Sodium Peroxide, Na2O2
It is formed by heating sodium more than air free from moisture and carbon dioxide or over pure oxygen. Properties2Na 2O2 + 2H2O ——→ 4NaOH + O2; Na 2O2 + H2SO4 ——→ Na 2SO4 + H2O2
Properties2Na 2O2 + 2H2O ——→ 4NaOH + O2; Na 2O2 + H2SO4 ——→ Na 2SO4 + H2O2
3. Sodium Hydroxide (Caustic Soda), NaOH
It is one of the important chemicals and is manufactured on a very large scale forming an important chemical industry. It is most conveniently manufactured by one of the following processes:
(a) Methods involving sodium carbonate as a starting material: Two methods are used. These are
(i) Causticisation process (Gossage process)
Na2Co3 + Ca(Oh)2 ⇆ CaCo3 + 2NaOH
(ii) Lowig’s process
Na2CO3 + Fe2O3 ——→ 2NaFe2 + CO2; 2Na2FeO2 + H2O ——→ 2NaOH + Fe2O3
(b) Methods involving sodium chloride as starting material (electrolysis of brine)
At Cathod 2H2O + 2e ⇆ H2 + 2OH- ; Na+ + OH- ⇆ NaOH
At anode 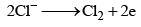
Properties:
- Strong alkali: NaOH ⇆ Na+ + OH-
(i) NaOH + HCl ——→ NaCl + H2O;
(ii) 2NaOH + CO2 ——→ Na2CO3 + H2O
(iii) Al2O3 + 2NaOH ——→ 2NaAlO2 + H2O - Action on non-metals Cl2 + 2NaOH ——→ NaCl + NaClO + H2O
3Cl2 + 6NaOH ——→ 5NaCl + NaClO3 + 3H2O
P4 + 3NaOH + 3H2 O ——→ 3NaH2PO2 + PH3 - Action on Metals Zn + 2NaOH ——→ Na2ZnO2 + H2;
- Action on Salts
(i) Ni(NO3)2 + 2NaOH ——→ Ni(OH)2 + 2NaNO3
(ii) Insoluble hydroxides which dissolve more than NaOH
ZnSO4 + 2NaOH ——→ Zn(OH)2 + Na2SO4
(iii) Unstable hydroxides 2AgNO3 + 2NaOH ——→ 2AgOH + 2NaNO3
2AgOH ——→ Ag2O + H2O
4. Sodium Carbonate or Washing Soda (Na2CO3.10H2O)
(a) Le-Blanc Process
(i) Conversion of NaCl into
Na2CO4 NaCl + H2SO4 ——→ NaHSO4 + HCl
NaHSO4 + NaCl ——→ Na2SO4 + HCl
(b) Solvay ammonia soda process
NH3 + H2O + CO2 ——→ NH4HCO3
NaCl + NH4HCO3 ——→ NaHCO3 + NH4Cl
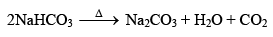
Chemical Properties of Sodium Carbonate
- Action of acids
Na2CO3 + HCl → NaHCO3 + NaCl; NaHCO3 + HCl → NaCl + H2O + CO2 - The action of water & carbon dioxide
Na2CO3 + H2O + CO2 → 2NaHCO3 - Action of silica
When the mixture of sodium carbonate and silica is fused, sodium is formed. Sodium silicate is called soluble glass or water glass as it is soluble in water.
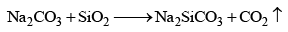
- Action on salt of non-alkali metals
The solution of carbonates reacts with metal salts (except alkali metal Salt) to form insoluble normal or basic carbonates.
2MgCl2 + 2Na2CO3 + H2O → MgCO3 · Mg(OH)2 + 4NaCl + CO2
5. Sodium Bicarbonates (NaHCO3)
Na2CO3 + CO2 + H2O → 2NaHCO3 (Sparingly soluble)
Properties
NaHCO3 + H2O → NaOH + H2CO3; 2NaHCO3 → Na2CO3 + H2O + CO2
6. Sodium thiosulphate (Na2S2O3.5H2O)
(i) Spring’s reaction Na2S + I2 + Na2SO3 → Na2S2O3 + 2NaI
Properties
(i) Oxidation
2Na2S2O3 + I2 → 2NaI + Na2S4O6
7. Sodium ammonium hydrogen phosphate
(NaNH4HPO4)
Preparation
NH4Cl + Na2HPO4 + 4H2O → NaNH4HPO4.4H2O + NaCl
Properties (i) Na(NH4)HPO4 → NaPO3 + NH3 + H2O
8. Sodium Cyanide (NaCN)
From calcium cyanide
Ca(CN)2 + C + Na2CO3 → 2NaCN + CaCO3
Properties:
It forms complex cyanides with the salts of copper cadmium, zinc, iron, cobalt, nickel etc.
Some examples are given below.
(i) AgNO3 + NaCN → AgCN + NaNO3; AgCN + NaCN → Na[Ag(CN)2]
Compounds of Potassium
Carnalite KClMgCl2 6H2O
Kainite KCl.MgSO4.MgCl2.3H2O
Indian Saltpetre KNO3
Felspar K2O.Al2O3.6SiO2 (clay)
Polyhalite K2SO4MgSO4.CaSO4.6H2O
Sylvine KCl
Potassium Iodide (KI)
Preparation(i) KOH + HI → KI + H2O
(ii) 3I2 + 6KOH → 5KI + KIO3 + 3H2O
Complex Formations
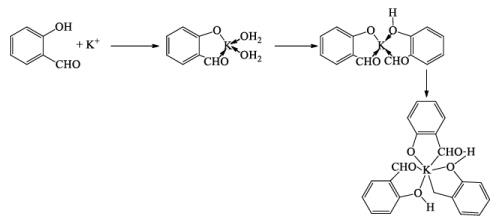
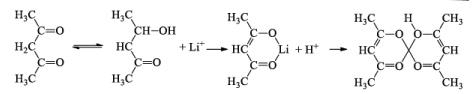
- An unusual compound [Na(Cryptanad-222)]+ Na– has an interesting feature in that it contains Na–, the iodide ion (Negative charge on metal).
|
48 videos|92 docs|41 tests
|
FAQs on Chemical & Physical Properties of Alkali Metals - Inorganic Chemistry
| 1. What is the electronic configuration of alkali metals? |  |
| 2. How do ionization energies of alkali metals vary across the periodic table? |  |
| 3. What is the significance of electropositive character in alkali metals? |  |
| 4. What are some common compounds of sodium? |  |
| 5. How do the physical properties of alkali metals contribute to their chemical reactivity? |  |

|
Explore Courses for Chemistry exam
|

|
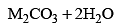 ⇆
⇆