Short & Long Answer Questions: Thermal Properties of Matter - 2 | Physics for JAMB PDF Download
Q.51 Why juice bottles are placed under water in the cold countries?
Answer: This is done so to prevent the freezing of juice. Water has to release comparatively large amount of heat to lower its temperature to the same extent than juice and hence the chances of freezing are reduced.
Q.52 Why is water used as an effective coolant?
Answer: The specific heat of water is very high. When it runs over hot parts of an engine or machinery, it absorbs a large amount of heat. This helps in maintaining the temperature of the engine low.
Q.53 What kind of thermal conductivity and specific heat requirements would you specify for cooking utensils?
Answer: A cooking utensil should have (i) high conductivity so that it can conduct heat through itself and transfer it to the contents quickly, (ii) low specific heat so that it immediately attains the temperature of the source.
Q.54 Why do the metal utensils have wooden handles?
Answer: Wood is a bad conductor of heat. Wooden handle does not allow heat to be conducted from the hot utensil to the hand. So we can easily hold the hot utensil with its help.
Q.55 Why birds are often seen to swell their feathers in winter?
Answer: When the birds swell their feathers, they are able to enclose air in the feathers. Air, being a poor conductor of heat, prevents the loss of heat from the bodies of the birds to the surroundings and as such they do not feel cold in winter.
Q.56 Why an ice box is constructed with a double wall?
Answer: An ice-box is made of double wall, and the space between the walls is filled with some non- conducting material to provide heat insulation, so that the loss of heat can be minimised.
Q.57 Why are two thin blankets are warmer than a single blanket of double the thickness?
Answer: The air enclosed between two blankets prevents the transfer of heat from our body to outside. Thus it provides a better insulation than a single blanket of double thickness.
Q.58 A squirred wraps its bushy tail round its body during its winter sleep. Why?
Answer: The bushy tail provides its body a non- conducting blanket. So the loss of heat by conduction is minimized.
Q.59 Calorimeters are made of metals not glass. Why?
Answer: This is because metals are good conductors of heat and have low specific heat capacity.
Q.60 When we step barefoot into an office with a marble floor, we feel cold. Why?
Answer: This is because marble is a better conductor of heat than concrete. When we walk barefooted on a marble floor, heat flows our body through the feet and we feel cold.
Q.61 Why we can easily boil water in a paper cup?
Answer: This is because heat is easily conducted through the paper to the water, and as such the temperature attained is not sufficient for the paper to be charred.
Q.62 A piece of paper wrapped tightly on a wooden rod is observed to get charred quickly when held over a flame as compared to a similar piece of paper when wrapped on a brass rod. Explain why.
Answer: Brass is a good conductor of heat. It quickly conducts away the heat. So, the paper does not alter its ignition point easily. On the other hand, wood is a bad conductor of heat and is unable to conduct away the heat. So, the paper quickly reaches its ignition point and is charred.
Q.63 A piece of wire gauze is placed over the Bunsen burner. If the gas is turned on below the gauge, will the flame go above the gauge?
Answer: Copper is a very good conductor of heat. The copper gauze absorbs most of the heat. So the temperature of the gas above the gauze does not reach its ignition temperature.
Q.64 Woolen clothes are worn in winter. Why?
Answer: Woolen fibres enclose a large amount of air in them. Both wool and air are bad conductors of heat. The small coefficient of thermal conductivity prevents the loss of heat from our body due to conduction. So we feel warm in woolen clothes.
Q.65 Why do we use copper gauze in Davy's safety lamp?
Answer: In Davy's safety lamp used in mines, a copper gauze is placed around the flame of the lamp. Since the copper gauze is good conductor of heat, it absorbs heat of the flame. This keeps the temperature outside the copper gauze less than the ignition temperature and so the marsh gas does not catch fire.
Q.66 Place a safety pin on a sheet of paper. Hold the sheet over a burning candle, until the paper becomes yellow and charr. On removing the pin, its white trace is observed on the paper. Why?
Answer: The safety pin is made of steel which is good conductor of heat. So the safety pin takes heat from the paper under it and transfers it away to the surroundings. The portion of the paper under the safety pin remains comparatively colder than the remaining part.
Q.67 Stainless steel cooking pans are preferred with extra copper bottom. Why?
Answer: The thermal conductivity of copper is much larger than that of steel. The copper bottom allows more heat to flow into the pan and hence helps in cooking the food faster.
Q.68 If a drop of water falls on a very hot iron, it does not evaporate for a long time. Give reason.
Answer: When a drop of water falls on a very hot iron, it gets insulated from the iron by a layer of poor conducting water vapour. As the heat is conducted very slowly through this layer, it takes quite long for the drop to evaporate. But if the drop of water falls on iron which is not very hot, then it comes in direct contact with iron and evaporates immediately.
Q.69 Pieces of copper and glass are heated to the same temperature. Why does the piece of copper feel hotter on touching?
Answer: Copper is much better conductor of heat than glass. When we touch the hot copper piece, heat readily flows to our hand. But this is not the case when the hot glass piece is touched.
Q.70 Usually a good conductor of heat is a good conductor of electricity also. Give reason.
Answer: Electrons contribute largely both towards the flow of electricity and the flow of heat. A good conductor contains a large number of free electrons. So it is both a good conductor of heat and electricity.
Q.71 Why do electrons in insulators not contribute towards its thermal conductivity?
Answer: Insulators do not have free electrons inside them. So electrons have no contribution towards their thermal conductivity.
Q.72 Why felt rather than air is employed for thermal insulation?
Answer: Though air is a bad conductor of it, it transfers heat easily by convection. Felt traps air between its fibres and convection currents cannot be set up in it. This makes felt a better thermal isolator than air.
Q.73 If air is poor conductor of heat, why do we not feel warm without clothes?
Answer: Although air is poor conductor of heat, it carries away heat from body due to convection when we are without clothes. Hence we feel cold.
Q.74 Why small holes are provided at the bottom of the chimney of the lamp?
Answer: The hot air and burnt gases rise upwards through the chimney. Fresh air enters through the holes provided at the bottom. In the absence of these holes, convection currents will not be set up and the lamp would go off.
Q.75 Why rooms are provided with the ventilators near the roof?
Answer: It is done so to remove the harmful impure air, and to replace it by the cool fresh air. The air we breath out is warm and so it is lighter. It rises upwards and can go out through the ventilator provided near the roof. The cold fresh air from outside enters the room though the doors and windows. Thus the convection current is set up in the air.
Q.76 Why it is much hotter above a fire than by its side?
Answer: Heat carried away from a fire sideways mainly by radiation. Above the fire, heat is carried by both radiation and convection of air. But convection carries much more heat than radiation. So it is much hotter above a fire than by its sides.
Q.77 Why snow is a better heat insulator than ice?
Answer: When the temperature of the atmosphere reaches below 0∘C, the water vapours present in air freeze directly in the form of minute particles of ice. Many particles combine and take cotton-like shape which is called snow. Snow contains a large number of air pockets which prevent the formation of convection currents. Hence snow acts as a good heat insulator than ice.
Q.78 Can we boil water inside an earth satellite?
Answer: No. The process of transfer of heat by convection is based on the fact that a liquid becomes lighter on becoming hot and rises up. In condition of weightlessness, this is not possible. So transfer of heat by convection is not possible in a satellite.
Q.79 Water is heated from below. Why?
Answer: When water is heated, its density decreases and it rises up. Cooler liquid of the upper part takes its place and so convection currents are set up and water gets heated up. If heated from the top, it will conduct very small amount of heat to the bottom because water is poor conductor of heat.
Q.80 Suppose you want to cool your drink. Should you keep ice cubes floating on the top or should you arrange to keep the ice cubes at the bottom?
Answer: Ice cubes should be kept floating in the drink. The liquid will then cool by convection. If the ice cubes are placed at the bottom, no convection currents are set up and liquid is not cooled. Also it cannot be cooled by conduction because liquid is a poor conductor of heat.
Q.81 Why are the cooling coils fitted near the ceiling of a refrigerator?
Answer: As the air gets cooled in the upper part of the refrigerator, it becomes denser and goes down. The warmer air of the lower part moves up. Thus convection currents are set up. This quickly cools up the entire inside of the refrigerator.
Q.82 After some time of the switching on an electric heater, the temperature of the heater becomes constant although current remains continuously flowing in it, why so?
Answer: When the steady state is reached, the rate of loss of heat by conduction, convection and radiation becomes equal to the rate of production of heat in the heater due to the flow of current.
Q.83 The earth constantly receives heat radiation from the sun and gets warmed up. Why does the earth not get as hot as the sun?
Answer: Because the earth is located at a very large distance from the sun, hence it receives only a small fraction of the heat radiation emitted by the sun. Further, due to loss of heat from the surface of earth due to convection and radiation also, the earth does not become as hot as the sun.
Q.84 Why do animals curl into a ball, when they feel very cold?
Answer: The total energy radiated by a body depends on its surface area. Thus when the animals feel very cold, they curl their bodies into a ball so as to decrease the surface area of their bodies which in turn helps to reduce the amount of heat lost by them.
Q.85 Two thermos flasks are of the same height and same capacity. One has a circular cross section while the other has a square cross-section. Which of the two is better?
Answer: As both flasks have same height and capacity, the area of the cylindrical wall will be less than that of the square wall. Hence the thermos flask of circular cross-section will transmit less heat as compared to the thermos flask of square cross-section and will be better.
Q.86 Why a body with large reflectivity is a poor emitter?
Answer: A body whose reflectivity is large would naturally absorb less heat. So, a body with large reflectivity is a poor emitter.
Q.87 Why does a piece of red glass when heated and taken out glow with green light?
Answer: At low temperature, the red glass absorbs green colour strongly. But at higher temperatures, it emits green colour strongly.
Q.88 Two stars radiate maximum energy at wavelengths 3.6×10−7 m and 4.8×10−7m respectively. What is the ratio of their temperatures?
Answer: Here
λm=3.6×10−7m, λ′m=4.8×10−7m
By Wien's law, λmT=λ′mT′ ∴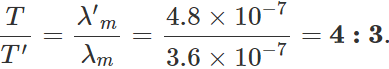
Q.89 If all the objects radiate electromagnetic energy, why do not the objects around us in everyday life become colder and colder?
Answer: According to the Prevost theory of heat exchanges, all the objects (above 0 K) not only radiate electromagnetic energy but also absorb at the same rate from their surroundings. Thus they do not become colder.
Q.90 Is it necessary that all black coloured objects should be considered black bodies?
Answer: No. A polished black surface is not a black body because it reflects radiation incident on it. On the other hand, the sun, which is a dazzling white body, is a black body.
Q.91 Why are clear nights colder than cloudy nights?
Answer: Clouds are opaque to heat radiations. So on a cloudy night, radiations from the earth's surface fail to escape. But on a clear night, the surface of the earth is cooled due to excessive radiation. So a clear night is colder than a cloudy night.
Q.92 White clothes are more comfortable in summer while colourful clothes are more comfortable in winter. Why?
Answer: White clothes absorb very little heat radiation and hence they are comfortable in summer. Coloured clothes absorb almost whole of the incident radiation and keep the body warm in winter.
Q.93 Explain why cooking utensils are often blackened at the bottom and polished at the top.
Answer: Black surfaces are good absorbers of heat radiations. The bottom of the cooking utensils is blackened so that it absorbs maximum heat radiations. Polished white surfaces are bad absorbers and hence bad emitters of heat radiations. By polishing the upper parts of the cooking utensils, the loss of heat by radiation is minimized.
Q.94 Gasoline tanks are generally painted with aluminium paint. Why?
Answer: The shining aluminium paint is a bad absorber of heat. So the tank painted with aluminium paint on the outside is prevented from getting excessively heated in the sun.
Q.95 A hole in the cavity of a radiator is a black body. Why?
Answer: A hole in the cavity of a radiator does not reflect any radiation and absorbs all the radiation incident on it. So it is a black body.
Q.96 Why is there the word displacement in Wien's displacement law?
Answer: As the temperature is increased, the wavelength having maximum intensity is displaced towards the shorter wavelength region. Hence the word displacement is used.
Q.97 Black body radiation is white. Comment.
Answer: True. A black body absorbs radiations of all wavelengths. When heated to a suitable temperature, it emits radiations of all wavelengths. Hence a black body radiation is white.
Q.98 Which object will cool faster when kept in open air, the one at 300∘ C or the one at 100∘ C? Why?
Answer: The object at 300∘C will cool faster than the object at 100∘C. This is in accordance with Newton's law of cooling, Rate cooling of an object ∝ Temperature between the object and its surroundings
Q.99 In what respect is the thermal radiation different from light?
Answer: Thermal radiations are electromagnetic waves having wavelength range from 1μm to 100μm. When they are absorbed by a body, they produce heat. Light radiations are electromagnetic waves having wavelength range from 4000 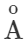 to 7500
to 7500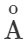 . They produce sensation of vision.
. They produce sensation of vision.
Q.100 What is critical temperature?
Answer: It is the temperature of a substance in the gaseous state below which the gas can be liquified by pressure only, and above which the gas cannot be liquified. This implies that gas is simply a vapour below its critical temperature = λ'mТ
Q.101 Can a gas be liquified at any temperature by the increase of pressure alone?
Answer: No. A gas can be liquified by pressure alone, only when its temperature is below its critical temperature.
Q.102 What is the effect of pressure on melting point of a solid?
Answer: The melting point of a solid may increase or decrease depending on the nature of solid. For solids such as ice which contracts on melting, it is lowered while for solids such as sulphur and wax which expand on melting it increases.
Q.103 How does the boiling point of water change with pressure?
Answer: The boiling point of water increases with the increase in pressure.
Q.104 What is the temperature above which steam will not condense to water even if it is compressed (isothermally) to very large pressure?
Answer: Above the critical temperature (374.1∘C for water), steam will not condense to water.
Q.105 What is the significance of negative slope of ice line of water?
Answer: It indicates that the melting point of ice decreases with increase in pressure (on ice line). This is because volume of water formed on melting is less than the volume of ice (before melting).
Q.106 Are the relative amounts of ice, water and vapour fixed at the triple point water?
Answer: At the triple-point of water, the temperature and pressure are fixed. However, the relative amounts of the three phases are not unique. The relative amounts of the three phases can be varied by adding or taking out heat from the system.
Q.107 What happens if water vapour at a pressure of 0.004 atm is cooled to 0∘C?
Answer: The pressure corresponding to triple point is 610 Pa which is equal to nearly 0.006 atm. It follows from the P-T phase diagram of water that at a pressure lower than this pressure, water vapour condenses directly to ice without passing through the liquid phase.
Q.108 Water exists in liquid phase at 30∘C at 1 atmospheric pressure. How would you convert this water to vapour form without increasing its temperature?
Answer: Water at 30∘C can be converted into vapour by reducing its pressure until it equals the vapour pressure of water at 30∘C. This route is along a vertical line on the P-T diagram, with T = 30∘C.
Q.109 What are the critical temperature and pressure for CO2? What is their significance?
Answer: The critical temperature and pressure of CO2 are 31.1∘C and 73.0 atm respectively. Above this temperature, CO2 will not liquify even if compressed to high pressures.
Q.110 Ice of 0∘ is converted into steam at 100° C. State the isothermal changes in the process.
Answer: The isothermal changes are (i) Conversion of ice at 0∘C into water at 0∘C. (ii) Conversion of water at 100∘C into steam at 100∘C.
Q.111 Explain why a new quilt is warmer than an old one.
Answer: A new quilt contains more air in its pores as compared to the old quilt. As air is bad conductor of heat, it does not allow heat to be conducted away from our body to the surroundings and we feel warmer in it.
Q.112 Give reasons why water is considered unsuitable for use in thermometers.
Answer: Water is considered unsuitable for use in thermometers due to following reasons:
(i) The expansion of water with temperature is non-uniform.
(ii) Due to its large specific heat and low thermal conductivity, a water thermometer does not respond to changes in temperature quickly.
(iii) Water is invisible, sticks to glass and has high rate of evaporation.
(iv) Its temperature range is small from 0∘C to 100∘C
Q.113 Give four reasons why is mercury used in thermometers.
Answer: The reasons for using mercury in a thermometer are
(i) Mercury has a uniform expansion over a wide range of temperature.
(ii) Mercury is opaque and bright, so it can be easily seen in a glass tube.
(iii) It does not stick to the walls of the glass tube.
(iv) It is a good conductor of heat and has low thermal capacity.
Q.114 Give reasons why is a platinum wire used in a resistance thermometer.
Answer: The reasons for using a platinum wire in a resistance thermometer are:
(i) The resistance of a platinum wire increases uniformly with the rise in temperature (from 200∘C to 1200∘C).
(ii) It does not react chemically with other substances
(iii) Its melting point is quite high (1800∘C).
Q.115 Give some merits of gas thermometers over those of mercury thermometers.
Answer: Some merits of gas thermometers are
(i) A gas thermometer is more sensitive than a mercury thermometer,
(ii) The working of a gas thermometer is independent of the nature of the gas used.
(iii) A gas thermometer can measure very low and very high temperatures.
Q.116 Name the suitable thermometers to measure the following temperatures: - 80∘ C, 60∘ C, 250∘ C, 780∘ C, 2000∘ C
Answer: Gas thermometer for -80∘C. Mercury thermometer for 60∘C. Platinum resistance thermometer for 250∘C and 780∘C. Total radiation pyrometer for 2000∘C.
Q.117 Suggest suitable methods for measuring the temperature (i) surface of the sun, (ii) surface of the earth, (iii) an insect, and (iv) liquid helium.
Answer: (i) By using total radiation pyrometer. (ii) By using a thermoelectric thermometer by embedding its hot junction in the earth. (iii) By using a thermoelectric thermometer by touching its hot junction with the insect. (iv) By using a magnetic thermometer which is based on Curie's law: the susceptibility of a paramagnetic material varies inversely with its absolute temperature.
Q.118 In problem 7, will the distance between the two holes increase or decrease on heating?
Answer: When the metal sheet is heated, it expands a whole. Therefore, the holes will increase in diameter as well as move outwards. The distance BC between the two holes increases.
Q.119 There are two spheres of same radius and material at same temperature but one being solid while the other hollow. Which sphere will expand more if (i) they are heated to the same temperature (ii) same amount of heat is given to each of them?
Answer: (i) As thermal expansion of isotropic solids is similar to true photographic enlargement, the expansion of a cavity is same as if it were a solid body of the same material i.e., 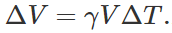 .As here
.As here 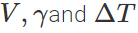 are same for both solid and hollow spheres, so the expansions of both will be equal. (ii) If same amount of heat is given to the two spheres, then due to lesser mass, rise in temperature of hollow sphere will be more (as ΔT=Q/Mc) and hence the expansion will be more as
are same for both solid and hollow spheres, so the expansions of both will be equal. (ii) If same amount of heat is given to the two spheres, then due to lesser mass, rise in temperature of hollow sphere will be more (as ΔT=Q/Mc) and hence the expansion will be more as 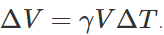
Q.120 Two bodies of specific heats c1 and c2 having same heat capacities are combined to form a single composite body. What is the specific heat of the composite body?
Answer: As the heat capacities are equal, so m1c1=m2c2. Let c be the specific heat of the composite body. Then (m1+m2)c=m1c1+m2c2=m1c1+m1c1=2m1c1 or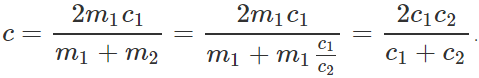
Q.121 Two rods A and B are of equal length. Each rod has its ends at temperatures T1 and T2 . What is the condition that will ensure equal rates of flow of heat through the rods A and B?
Answer: Let x be the length of each rod. The rates of flow of heat through the rods A and B will be equal if 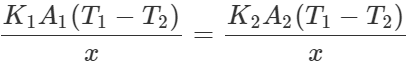 or
or 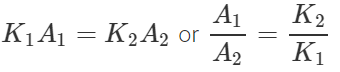 Hence for equal rates of flow of heat, the areas of cross-section of the two rods should be inversely proportional to their coefficients of thermal conductivity.
Hence for equal rates of flow of heat, the areas of cross-section of the two rods should be inversely proportional to their coefficients of thermal conductivity.
Q.122 Two vessels of different materials are identical in size and wall-thickness. They are filled with equal quantities of ice at 0∘ C. If the ice melts completely in 10 and 25 minutes respectively, compare the coefficients of thermal conductivity of the materials of the vessels.
Answer: Let K1 and K2 be the coefficients of thermal conductivity of the materials and t1 and t2 be the times in which ice melts in the two vessels. As the same quantity of ice melts in the two vessels, the quantity of heat flowed into the vessels must be same. ∴
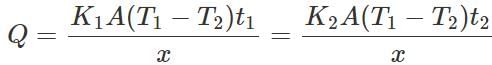 or K1t1=K2t2 ∴
or K1t1=K2t2 ∴
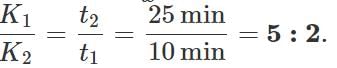
Q.123 Two vessels A and B of different materials but having identical shape, size and wall- thickness are filled with ice and kept at the same place. Ice melts at the rate of 100gmin−1 and 150gmin−1 in A and B respectively. Assuming that heat enters the vessels through the walls only, calculate the ratio of thermal conductivities of their materials.
Answer: Let m1 and m2 be the masses of ice melted in same time t (=1 min)in vessels A and B respectively. Then the amounts of heat flowed into the two vessels will be 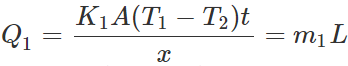 ?(i)
?(i) 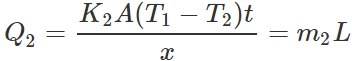 ?(ii) where L is latent heat of ice. Dividing (i) by (ii), we get
?(ii) where L is latent heat of ice. Dividing (i) by (ii), we get 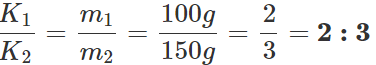
Q.124 A sphere, a cube and a thin circular plate, all made of the same material and having the same mass are initially heated to a temperature of 200∘C. Which of these objects will cool fastest and which one slowest when left in air at room temperature? Give reasons.
Answer: The thin circular plate has the largest surface area. The sphere has the smallest surface area. Thus the plate will radiate maximum heat while the sphere will radiate minimum heat. Hence the plate will cool fastest and the sphere will cool slowest.
Q.125 There are two rods of the same metal, same length, same area of cross-section, but one of square cross-section and the other of circular cross- section. One end of each is kept immersed in steam. After the steady state is reached, the other ends of the rods are touched. Which one will be hotter? Give reason.
Answer: The surface area of the rod of circular cross-section will be smaller, because for a given area a circle has least perimeter. So the loss of heat by radiation will be small. Hence the other end of the circular rod will be hotter than the other end of the square rod.
Q.126 A solid sphere of copper of radius R and a hollow sphere of the same material of inner radius r and outer radius R are heated to the same temperature and allowed to cool in the same environment. Which of them starts cooling faster?
Answer: Rate of loss of heat by any sphere, 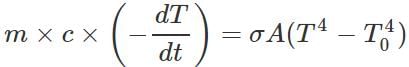 Now σ,A,(T4−T40) are same for both the spheres, so the rate of cooling,
Now σ,A,(T4−T40) are same for both the spheres, so the rate of cooling, 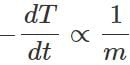 Since the hollow sphere has less mass, its rate of cooling will be faster.
Since the hollow sphere has less mass, its rate of cooling will be faster.
Q.127 On a hot day, a car is left in sunlight with all the windows closed. After some time, it is found that the inside of the car is considerably warmer than the air outside. Explain, why.
Answer: Glass transmits about 50% of heat radiation coming from a hot source like the sun but does not allow the radiation from moderately hot bodies to pass through it. Due to this, when a car is left in the sun, heat radiation from the sun gets into the car but as the temperature inside the car is moderate, they do not pass back through its windows. Hence, inside of the car becomes considerably warmer.
Q.128 How does tea in a thermos flask remain hot for a long time?
Answer: The air between the two walls of the thermos flask is evacuated. This prevents heat loss due to conduction and convection. The loss of heat due to radiation is minimized by silvering the inside surface of the double wall. As the loss of heat due to the three processes is minimized, the tea remains hot for a long time.
Q.129 Distinguish between conduction, convection and radiation.
Answer:
| Conduction | Convection | Radiation | |
| 1. | Material medium is required. | Material medium is required. | No material medium is required. |
| 2. | It is due to temperature difference. Heat flows from high temperature region to low temperature region. | It is due to difference in density. Heat flows from low density region to high density region. | It occurs from all bodies at temperatures above 0 K. |
| 3. | It occurs in solids through molecular collisions, without actual flow of matter. | It occurs in fluids by actual flow of matter. | It can take place at large distances and does not heat the inter- vening medium. |
| 4. | It is a slow process. | It is also a slow process. | It propagates at the speed of light. |
| 5. | It does not obey the laws of reflection and refraction. | It does not obey the laws of reflection and refraction. | It obeys the laws of reflection and refraction. |
Q.130 A blackened platinum wire, when gradually heated, first appears dull red, then blue and finally white. Explain why.
Answer: According to Wien's displacement law, when blackened platinum wire is gradually heated, it first emits radiations of longer wavelengths, so it appears red. At higher temperatures, it emits blue radiations more strongly than red and appears blue. At very high temperatures, it emits all radiations strongly and appears white.
Q.131 In a coal fire, the pockets formed by coals appear brighter than the coals themselves. Is the temperature of such a pocket higher than the surface temperature of a glowing coal?
Answer: The temperature of pockets formed by coals are not appreciably different from the surface temperatures of glowing coals. However, the pockets formed by coals act as cavities. The radiations from these cavities are black body radiations and so have maximum intensity. Hence the pockets appear brighter than the glowing coals.
Q.132 Answer the following questions : (a) A vessel with a movable piston maintained at a constant temperature by a thermostat contains a certain amount of liquid in equilibrium with its vapour. Does this vapour obey Boyle's law? In other words, what happens when the volume of vapour is decreased? Does the vapour pressure increase? (b) What is meant by 'superheated water' and 'super cooled vapour'? Do these states of water lie on its P-V-T surface? Give some practical applications of these states of water.
Answer: (a) No, the vapour in equilibrium with its liquid does not obey Boyle's law. When the volume of the vapour is decreased by applying pressure, some of the vapours condense into liquid, maintaining the same pressure of the vapour at the given temperature i.e., vapour pressure does not increase when the volume of vapours is decreased.
(b) Superheated water. Water in liquid phase having temperature above the boiling point of water at the given pressure is called superheated water. It is highly unstable stage. Super cooled vapour. Water in vapour phase having temperature below its boiling point at the given pressure is called super cooled vapour. It is also highly unstable stage. As the above states of water are not equilibrium states, so they do not lie on P-V-T surface of water. Applications. These unstable states of water are used in bubble chamber and cloud chamber for detecting high speed charged particles.
|
263 videos|252 docs|237 tests
|
FAQs on Short & Long Answer Questions: Thermal Properties of Matter - 2 - Physics for JAMB
| 1. What is thermal expansion? |  |
| 2. How is thermal conductivity defined? |  |
| 3. What is specific heat capacity? |  |
| 4. How does thermal radiation work? |  |
| 5. What is the difference between specific heat capacity and heat capacity? |  |
















