Structure of the Atom Class 9 Notes Science Chapter 4
| Table of contents |

|
| Atomic Structure |

|
| Thomson's model of an Atom |

|
| Rutherford's Model of an Atom |

|
| Bohr's Model of Atom |

|
| Distribution of Electrons in Different Orbits |

|
| Valency |

|
| Atomic Number & Mass Number |

|
The structure of an atom comprises protons, neutrons and electrons. These basic components provide the mass and charge of the atoms.
Atomic Structure
Atomic Structure refers to the arrangement of subatomic particles—protons, neutrons, and electrons—within an atom, defining its composition and behavior.
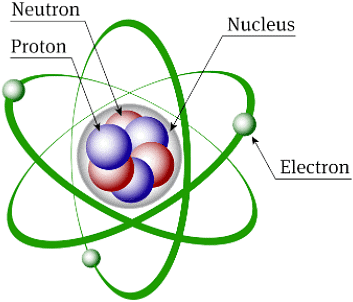 Structure of Atom
Structure of Atom
- John Dalton assumed that the atom is indivisible.
- In 1866 E. Goldstein discovered the presence of new radiations in a gas discharge tube and called them canal rays. These rays were positively charged radiations which led to the discovery of a sub-atomic particle called proton.
- In 1897 J.J. Thomson discovered the sub-atomic particle - the electron with a negative charge.
- The neutron was discovered by Chadwick. Neutrons have no charge.
- Table: Properties of Electrons, Neutrons and Protons

Thomson's model of an Atom
Thomson's Model of the Atom, known as the "plum pudding model," proposed that the atom is a sphere of positive charge with negatively charged electrons embedded throughout, like plums in a pudding.
- In the sphere of protons, electrons are distributed.
- The total positive charge in an atom is equal to the total negative charge.
- Atom is electrically neutral.
- Also known as the plum pudding model.
 Plum Pudding Model
Plum Pudding Model
Rutherford's Model of an Atom
Rutherford's Model of the Atom introduced the idea of a small, dense nucleus at the center of the atom, with electrons orbiting around it, fundamentally changing our understanding of atomic structure.
 Rutherford's Experiment
Rutherford's Experiment- α-Particles: (+2 charge and 4 mass) when fast-moving α-particles are bombarded on very thin gold foil, the following observations were made:
- Most of the α-particles passed straight through the gold foil.
- Some of the α-particles were deflected by the foil by small angles.
- One out of 12000 particles appeared to rebound.
Conclusions made by Rutherford
- Most of the space inside the atom is empty because most of the α-particles passed through the gold foil.
- Very few particles were deflected from their path because the +ve charge of the atom occupies very little space.
- A very small fraction of α-particles were rebounded back, showing all + ve charge and mass of the gold atoms were concentrated in a very small volume within the atom.
- The radius of the nucleus calculated was 10⁵ times less than the radius of the atom.
- Nuclear Model of an Atom given by Rutherford
(i) Centre → +ve charge → called nucleus. All mass resides in the nucleus.
(ii) Electrons → revolve around the nucleus in orbits.
(iii) The size of nucleus is very small compared to the size of the atoms.
 Rutherford's Nuclear Model of Atom
Rutherford's Nuclear Model of Atom
Drawbacks of Rutherford's model of the atom
- When an electron undergoes acceleration, it radiates energy. Thus revolving electrons would lose energy and finally fall into the nucleus.
- Due to this atoms should be highly unstable and hence matter would not exist in the form that we know.
- But we know that atoms are quite stable.
Bohr's Model of Atom
Bohr's Model of the Atom revolutionized our understanding of atomic structure by introducing the concept of electrons orbiting the nucleus in fixed energy levels, providing a clearer explanation of atomic stability and spectral lines.
Postulates of Neil Bohr
- Only special orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy.
- These orbits are called energy levels.
- Orbits or shells are represented by K, L, M, N or the numbers, n = 1, 2, 3, 4, .......
 Bohr's Model
Bohr's Model
Drawbacks of Bohr's Model of Atom
- It violates the Heisenberg Uncertainty Principle.
- It could not explain the spectra obtained from larger atoms.
Neutrons
In 1932, J. Chadwick identified a new subatomic particle with no electrical charge and a mass approximately equal to that of a proton. This particle was later called the neutron. Neutrons are found in the nuclei of all atoms except hydrogen. Typically, neutrons are represented by the symbol ‘n’. The total mass of an atom is determined by adding together the masses of the protons and neutrons in its nucleus.
Distribution of Electrons in Different Orbits
The distribution of electrons in different orbits, or energy levels, determines an atom's electron configuration and plays a key role in its chemical properties and reactivity
Rules
- Maximum number of electrons present in a shell is given by 2n2 (n = shell number)
Example: maximum number of electrons present in n = 1 are
n = 1 (K shell)
2(1)2 = 2 electrons - The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell unless the inner shells are completely filled.
Valency
Valency is the measure of an atom's ability to bond with other atoms, determined by the number of electrons in its outermost shell that can be gained, lost, or shared. Atomic structure of the first eighteen elements
Atomic structure of the first eighteen elements
- The combining capacity of an atom is called its valency.
- The number of bonds that an atom can form as part of a compound is expressed by the valency of the element.
- Valence electrons are those electrons that are present in the outermost orbit of the atom.
Atomic Number & Mass Number
The atomic number represents the number of protons in an atom's nucleus, while the mass number is the total count of protons and neutrons, providing insights into the atom's identity and mass.
- The total number of protons in the nucleus of an atom gives us the atomic number of that atom.
- It is represented with the letter ‘Z.’
Mass Number
- The number of protons and neutrons combined to give us the mass number of an atom.
- Mass Number is also called Atomic Mass.
- It is represented using the letter ‘A.’

An element is represented by AXZ, where Z is atomic number which is also equal to number of proton, A is mass number and X is symbol of the element. Mass number (A) = Number of protons (Z) + Number of neutrons.

Isotopes
- Atoms of the same element with the same atomic number but a different mass number, are called isotopes.
- Chemical properties → same
- Physical properties → different
 Isotopes of HydrogenApplications of Isotopes:
Isotopes of HydrogenApplications of Isotopes:
(a) An isotope of Uranium is used as fuel in nuclear reactors.
(b) An isotope of Cobalt is used in the treatment of cancer.
(c) An isotope of Iodine is used in the treatment of goitre.
Isobars
- Atoms of different elements with the same mass number but different atomic numbers are called isobars.
 Examples of Isobars
Examples of Isobars
Some Practice Questions:
Ques. How hydrogen atom is different from atoms of all other elements?
Ans. All the atoms are made up of three subatomic particles: electrons, protons and neutrons. Hydrogen atom is made up of only one electron and one proton. It does not contain any neutrons. So, it is different from atoms of all other elements.
Ques. What is mass number?
Ans. The mass number of an element is the sum of the number of protons and neutrons present in the atom of the element.
Mass number = No. of protons + No. of neutrons.
Example: A hydrogen atom has 1 proton but 0 neutron thus the mass number of H is 1.
The mass number of an element is denoted by the letter A. Protons and neutrons present in a nucleus, together known as nucleons.
Hence, Mass number = Number of nucleons.
Ques. What are the general features of isotopes?
Ans. The general features of isotopes are as follows:
1. The isotopes of an element have the same atomic number (i.e., the same number of protons in the nucleus and the same number of electrons in the extranuclear part)
2. The isotopes of an element have different mass numbers (i.e. different in the number of neutrons present in the nucleus)
3. Isotopes have the same electronic configuration and hence share similar chemical properties.
4. Isotopes of an element have different masses, so they have different physical properties like melting point, boiling point, density etc.
5. Due to differences in the nuclear structure (i.e., number of neutrons), they have different nuclear properties, e.g., the C-14 isotope is radioactive whereas the C-12 isotope is non-radioactive.
Ques. What are isotones?
Ans. Some atoms of different elements have different atomic numbers and different mass numbers but they have a same number of neutrons. These atoms are known as isotones.
Example:12C6 and 16O8.
Both C and O have the same number of neutrons i.e. 8.
|
87 videos|369 docs|67 tests
|
FAQs on Structure of the Atom Class 9 Notes Science Chapter 4
| 1. What is the difference between Thomson's, Rutherford's, and Bohr's models of an atom? |  |
| 2. How are electrons distributed in different orbits in an atom? |  |
| 3. What is valency in the context of atomic structure? |  |
| 4. How do the models of an atom help us understand the structure of matter? |  |
| 5. Why is the Rutherford model of an atom also known as the nuclear model? |  |

|
Explore Courses for Class 9 exam
|

|



















