Synthesis, Reactivity and Properties of Pyridine | Organic Chemistry PDF Download
What is Pyridine?
Pyridine is a heterocyclic compound which is colourless to yellow liquid with a chemical formula C5H5N.
It is a basic heterocyclic organic compound. It is also known as Azine or Pyridine. The structure is like benzene, with one methine group replaced by a nitrogen atom. It has a sour, putrid, and fish-like odour. Pyridine can be synthesized from ammonia, formaldehyde, and acetaldehyde or it can be made from crude coal tar.
It is weakly basic and is miscible with water. It is highly flammable and when inhaled or ingested it becomes toxic. Some symptoms, when exposed to pyridine, are nausea, asthmatic breathing, vomiting, headache, laryngitis, and coughing. It is widely used in the precursor to agrochemicals and pharmaceuticals. Also, it is used as an important reagent and organic solvent.
Synthesis
Historically, pyridine was extracted from coal tar or obtained as a byproduct of coal gasification. The process was labor-consuming and inefficient: coal tar contains only about 0.1% pyridine,and therefore a multi-stage purification was required, which further reduced the output. Nowadays, most pyridine is produced synthetically using various name reactions,
Chichibabin synthesis
The Chichibabin pyridine synthesis was reported in 1924 and is still in use in industry.In its general form, the reaction can be described as a condensation reaction of aldehydes, ketones, α,β-unsaturated carbonyl compounds, or any combination of the above, in ammonia or ammonia derivatives. In particular, unsubstituted pyridine is produced from formaldehyde and acetaldehyde, which are inexpensive and widely available. First, acrolein is formed in a Knoevenagel condensation from the acetaldehyde and formaldehyde. The acrolein is then condensed with acetaldehyde and ammonia to give dihydropyridine, which is oxidized with a solid-state catalyst to pyridine. This process is carried out in a gas phase at 400–450 °C. The product consists of a mixture of pyridine, simple methylated pyridines (picolines and lutidines); its composition depends on the catalyst used and can be adapted to the needs of the manufacturer. The catalyst is usually a transition metal salt such as cadmium(II) fluoride or manganese(II) fluoride, but cobalt and thallium compounds can also be used. The recovered pyridine is separated from byproducts in a multistage process.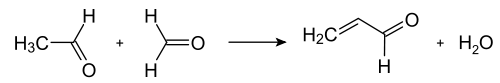
Formation of acrolein from acetaldehyde and formaldehyde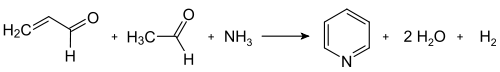
Condensation of pyridine from acrolein and acetaldehyde Practical applications of the traditional Chichibabin pyridine synthesis are limited by its consistently low yield, typically about 20%. This low yield, together with the high prevalence of byproducts, render unmodified forms of Chichibabin's method unpopular.
Reactivity
Despite the structural and bonding commonalities of benzene and pyridine, their reactivity differs significantly. Instead, in terms of its reactivity, pyridine more closely resembles nitrobenzene.
Electrophilic substitutions
Owing to the decreased electron density in the aromatic system, electrophilic substitutions are suppressed in pyridine and its derivatives. Friedel–Crafts alkylation or acylation, usually fail for pyridine because they lead only to the addition at the nitrogen atom. Substitutions usually occur at the 3-position, which is the most electron-rich carbon atom in the ring and is, therefore, more susceptible to an electrophilic addition.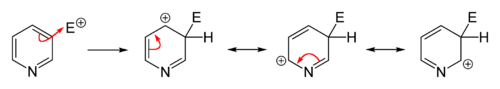
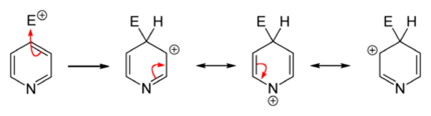
Direct nitration of pyridine is sluggish.Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination and chlorination of pyridine proceed well.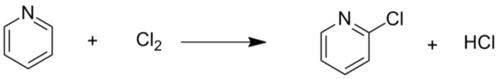
Pyridine-N-oxide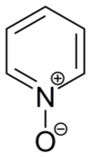
Structure of pyridine N-oxide
Oxidation of pyridine occurs at nitrogen to give pyridine-N-oxide. The oxidation can be achieved with peracids:
C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine-N-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Nucleophilic substitutions
In contrast to benzene ring, pyridine efficiently supports several nucleophilic substitutions. The reason for this is relatively lower electron density of the carbon atoms of the ring. These reactions include substitutions with elimination of a hydride ion and elimination-additions with formation of an intermediate aryne configuration, and usually proceed at the 2- or 4-position.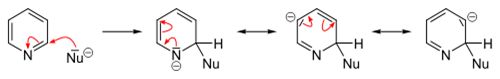
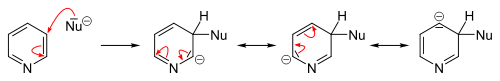
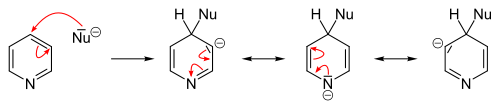
Many nucleophilic substitutions occur more easily not with bare pyridine but with pyridine modified with bromine, chlorine, fluorine, or sulfonic acid fragments that then become a leaving group. So fluorine is the best leaving group for the substitution with organolithium compounds. The nucleophilic attack compounds may be alkoxides, thiolates, amines, and ammonia (at elevated pressures).
In general, the hydride ion is a poor leaving group and occurs only in a few heterocyclic reactions. They include the Chichibabin reaction, which yields pyridine derivatives aminated at the 2-position. Here, sodium amide is used as the nucleophile yielding 2-aminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
Analogous to benzene, nucleophilic substitutions to pyridine can result in the formation of pyridyne intermediates as heteroaryne. For this purpose, pyridine derivatives can be eliminated with good leaving groups using strong bases such as sodium and potassium tert-butoxide. The subsequent addition of a nucleophile to the triple bond has low selectivity, and the result is a mixture of the two possible adducts.
Radical reactions
Pyridine supports a series of radical reactions, which is used in its dimerization to bipyridines. Radical dimerization of pyridine with elemental sodium or Raney nickel selectively yields 4,4'-bipyridine,or 2,2'-bipyridine, which are important precursor reagents in the chemical industry. One of the name reactions involving free radicals is the Minisci reaction. It can produce 2-tert-butylpyridine upon reacting pyridine with pivalic acid, silver nitrate and ammonium in sulfuric acid with a yield of 97%.
Reactions on the nitrogen atom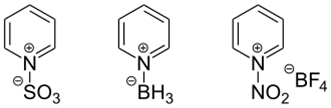
Additions of various Lewis acids to pyridine
Lewis acids easily add to the nitrogen atom of pyridine, forming pyridinium salts. The reaction with alkyl halides leads to alkylation of the nitrogen atom. This creates a positive charge in the ring that increases the reactivity of pyridine to both oxidation and reduction. The Zincke reaction is used for the selective introduction of radicals in pyridinium compounds (it has no relation to the chemical element zinc).
Hydrogenation and reduction
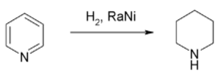
Reduction of pyridine to piperidine with Raney nickel
Piperidine is produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures.The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of benzene (205.3 kJ·mol−1).
Partially hydrogenated derivatives are obtained under milder conditions. For example, reduction with lithium aluminium hydride yields a mixture of 1,4-dihydropyridine, 1,2-dihydropyridine, and 2,5-dihydropyridine.Selective synthesis of 1,4-dihydropyridine is achieved in the presence of organometallic complexes of magnesium and zinc,and (Δ3,4)-tetrahydropyridine is obtained by electrochemical reduction of pyridine.
Properties of Pyridine
Pyridine and its simple derivatives are stable and relatively unreactive liquids, with strong penetrating odours that are unpleasant. Pyridine is the hydrogen derivative of this ring, it is benzene in which one CH- or methine group is replaced by a nitrogen atom. The structure of pyridine is completely analogous to that of benzene, being related by replacement of CH by N.
C5H5N | Pyridine |
Molecular Weight/ Molar Mass | 79.1 g/mol |
Density | 982 kg/m³ |
Boiling Point | 115 °C |
Melting Point | −41.6 °C |
|
44 videos|102 docs|52 tests
|
FAQs on Synthesis, Reactivity and Properties of Pyridine - Organic Chemistry
| 1. What is the synthesis of Pyridine? |  |
| 2. What is the reactivity of Pyridine? |  |
| 3. What are the properties of Pyridine? |  |
| 4. What are the applications of Pyridine? |  |
| 5. Is Pyridine toxic? |  |
















