Synthesis, Reactivity and Properties of Thiophene | Organic Chemistry PDF Download
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O) selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
Synthesis and Production
Reflecting their high stabilities, thiophenes arise from many reactions involving sulfur sources and hydrocarbons, especially unsaturated ones. The first synthesis of thiophene by Meyer, reported the same year that he made his discovery, involves acetylene and elemental sulfur. Thiophenes are classically prepared by the reaction of 1,4-diketones, diesters, or dicarboxylates with sulfidizing reagents such as P4S10 such as in the Paal-Knorr thiophene synthesis.
The Paal–Knorr Synthesis
The Paal- Knorr Synthesis in organic chemistry is a reaction that generates either furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes.
The furan synthesis requires an acid catalyst:
In the pyrrole synthesis a primary amine participates:

and in that of thiophene for instance the compound phosphorus pentasulfide: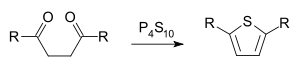
Thiophene synthesis
Paal-Knorr thiophene synthesis mechanism
Thiophene synthesis is achieved via a mechanism very similar to the furan synthesis. The initial diketone is converted to a thioketone with a sulfurizing agent, which then undergoes the same mechanism as the furan synthesis. Thiophene synthesis is achieved via a mechanism very similar to the furan synthesis. The initial diketone is converted to a thioketone with a sulfurizing agent, which then undergoes the same mechanism as the furan synthesis.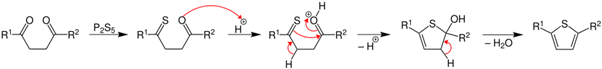
Most sulfurization agents are strong dehydrators and drive completion of the reaction. Early postulates toward the mechanism of the Paal-Knorr furan synthesis suggested that the thiophene was achieved by sulfurization of the furan product. Campaigne and Foye showed that treatment of isolated furans from the Paal-Knorr furan synthesis with phosphorus pentasulfide gave inconsistent results with the treatment of 1,4-dicarbonyls with phosphorus pentasulfide, which ruled out the sulfurization of a furan mechanism and suggests that the reaction proceeds via sulfurization of a dicarbonyl, producing a thioketone.
Reactions of Thiophene
Thiophene is considered to be aromatic, although theoretical calculations suggest that the degree of aromaticity is less than that of benzene. The "electron pairs" on sulfur are significantly delocalized in the pi electron system. As a consequence of its aromaticity, thiophene does not exhibit the properties seen for conventional sulfides. For example, the sulfur atom resists alkylation and oxidation.
Oxidation
Oxidation can occur both at sulfur, giving a thiophene S-oxide, as well as at the 2,3-double bond, giving the thiophene 2,3-epoxide, followed by subsequent NIH shift rearrangement.Oxidation of thiophene by trifluoroperacetic acid also demonstrates both reaction pathways. The major pathway forms the S-oxide as an intermediate, which undergoes subsequent Diels-Alder-type dimerisation and further oxidation, forming a mixture of sulfoxide and sulfone products with a combined yield of 83% (based on NMR evidence):
In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the isomer thiophene-2-one.Trapping experiments demonstrate that this pathway is not a side reaction from the S-oxide intermediate, while isotopic labeling with deuterium confirm that a 1,2-hydride shift occurs and thus that a cationic intermediate is involved.If the reaction mixture is not anhydrous, this minor reaction pathway is suppressed as water acts as a competing base.
Desulfurization by Raney nickel
Desulfurization of thiophene with Raney nickel affords butane. When coupled with the easy 2,5-difunctionalization of thiophene, desulfurization provides a route to 1,4-disubstituted butanes.
Polymerization
The polymer formed by linking thiophene through its 2,5 positions is called polythiophene. Polymerization is conducted by oxidation using electrochemical methods (electropolymerization) or electron-transfer reagents. An idealized equation is shown:
n C4H4S → (C4H2S)n + 2n H+ + 2n e−
Properties of Furan
At room temperature, thiophene is a colorless liquid with a mildly pleasant odor reminiscent of benzene, with which thiophene shares some similarities. The high reactivity of thiophene toward sulfonation is the basis for the separation of thiophene from benzene, which are difficult to separate by distillation due to their similar boiling points (4 °C difference at ambient pressure). Like benzene, thiophene forms an azeotrope with ethanol. The molecule is flat; the bond angle at the sulfur is around 93°, the C–C–S angle is around 109°, and the other two carbons have a bond angle around 114°.The C–C bonds to the carbons adjacent to the sulfur are about 1.34 Å, the C–S bond length is around 1.70 Å, and the other C–C bond is about 1.41 Å.
|
35 videos|92 docs|46 tests
|
FAQs on Synthesis, Reactivity and Properties of Thiophene - Organic Chemistry
| 1. What is the synthesis of thiophene? |  |
| 2. What are the reactivity patterns of thiophene? |  |
| 3. What are the properties of thiophene? |  |
| 4. How is the reactivity of thiophene influenced by substituents? |  |
| 5. What are some applications of thiophene derivatives? |  |

|
Explore Courses for Chemistry exam
|

|

















