Textbook Solutions: Separation of Substances | Eureka Plus Class 6: Book Solutions, Notes & Worksheets PDF Download
I. Tick (✔) the correct option.
1.
Ans: (c)
2.
Ans: (b)
3.
Ans: (c)
4.
Ans: (c)
5.
Ans: (c)
II. Classify the following as pure substances or mixtures.
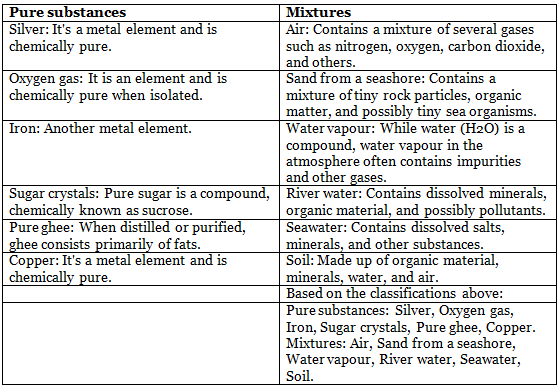
1.
Ans:
III. Write one word tor each of the following:
1.
Ans: Handpicking
2.
Ans: Sieving
3.
Ans: Winnowing
4.
Ans: Threshing
5.
Ans: Sedimentation
6.
Ans: Decantation
7.
Ans: Filtration
8.
Ans: Loading
IV. Answer the following questions in one sentence.
1.
Ans: A pure substance is entirely made of a single kind of substance.
2.
Ans: A mixture is a combination of different substances that are not chemically combined.
3.
Ans: Sieving.
4.
Ans: Solid substances that differ in size.
5.
Ans: Salt is obtained from seawater by the process of evaporation.
V. Answer the following questions in three to five sentences.
1.
Ans: Winnowing is a method of separating lighter and heavier solid substances from a mixture, such as chaff from grains. It involves dropping the mixture from a height, and wind carries away the lighter substances, leaving the heavier components behind.
2.
Ans: Immiscible liquids are those that do not mix and form separate layers, like oil and water. You can separate them using the process of decantation by pouring off one of the liquids without disturbing the other, or by using a separating funnel.
3.
Ans: To obtain salt from a mixture of salt, sand, and water, you can first use sedimentation and decantation to separate sand and water, and then you can use evaporation to remove the water, leaving behind the salt.
4.
Ans: Sedimentation is the process where undissolved solid particles settle at the bottom of a liquid. Decantation is carefully pouring off the liquid without disturbing the sediment. Loading is the process of adding a substance like alum to help settle tiny insoluble particles quickly.
5.
Ans: A saturated salt solution is a solution where no more salt can dissolve in the solvent at a particular temperature. When you heat a saturated solution, it can usually dissolve more solute (in this case, salt) because solubility typically increases with temperature.
6.
Ans: The separation of undissolved solids through sedimentation and decantation involves allowing a mixture of solid particles and liquid to stand undisturbed. During sedimentation, the heavier solid particles settle at the bottom (forming sediment), and then, during decantation, the liquid is gradually poured off without disturbing the settled solids, leaving behind the sediment. This process helps in separating the insoluble solid particles from the liquid.
Think and answer
1.
Ans: The surface of the Earth acts as a natural water filter. When rainwater falls, it percolates through layers of soil and rock, filtering out impurities and contaminants. Clean underground water is often the result of this natural filtration process. The water is purified as it travels through the Earth's layers, removing many impurities and providing a source of clean and freshwater.
2. Explain how you will separate each component of the mixtures mentioned below.
(a)
Ans: To separate a mixture of sand, shells, and water, you can use sedimentation and decantation. Let the mixture settle, and then carefully pour off the water while keeping the sand and shells at the bottom.
(b)
Ans: To separate a mixture of sand, shells, and salt, you can use water to dissolve the salt, leaving the sand and shells behind. Then, you can filter the solution to separate sand and shells from the saltwater.
(c)
Ans: To separate a mixture of oil, water, and sand, you can first use decantation to separate the oil from water, and then use sedimentation and filtration to separate the sand from the water.
|
22 videos|80 docs|16 tests
|
FAQs on Textbook Solutions: Separation of Substances - Eureka Plus Class 6: Book Solutions, Notes & Worksheets
| 1. How can filtration be used to separate substances? |  |
| 2. What is the process of evaporation used for in the separation of substances? |  |
| 3. How does magnetic separation work in the separation of substances? |  |
| 4. Why is distillation an effective method for separating substances? |  |
| 5. What is the purpose of chromatography in the separation of substances? |  |
|
22 videos|80 docs|16 tests
|

|
Explore Courses for Class 6 exam
|

|
















