Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > The Haber Process & Dynamic Equilibrium
The Haber Process & Dynamic Equilibrium | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| The Haber process - Higher |

|
| The Effect of Increasing Pressure |

|
| The Effect of Increasing Temperature |

|
| The Effect of Using a Catalyst |

|
| Choosing Reaction Conditions |

|
| Catalyst |

|
The Haber process - Higher
The Haber process involves a reversible reaction at dynamic equilibrium. The principles covered in Reversible reactions can be applied to explain how the rate and yield will be affected by the choice of reaction conditions.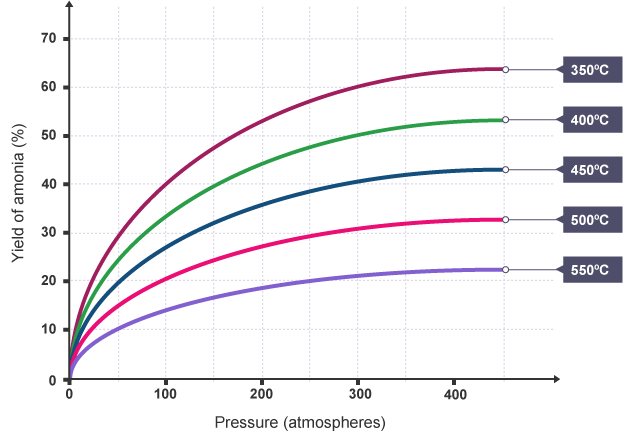 The yield of ammonia changes with changes in pressure and temperature
The yield of ammonia changes with changes in pressure and temperature
- This graph shows that for any specific temperature (ie following any of the coloured curved lines), as the pressure increases, so does the yield of ammonia.
- It also shows that at any given pressure (ie following a vertical line up from the x-axis), as the temperature decreases, the yield of ammonia increases. In other words, as the temperature increases, the yield decreases.
The Effect of Increasing Pressure
- In a reaction involving gases as reactants and/or products, increasing the pressure of the reaction mixture will cause the equilibrium position to move to the side with the fewest moles of gas, to reduce the pressure.
- There are fewer molecules on the right-hand side of the equation for the Haber process:
N2(g) + 3H2(g) ⇌ 2NH3(g)
1 + 3 = 4 molecules ⇌ 2 molecules - If the pressure is increased, the equilibrium position moves to the right, so the yield of ammonia increases. The rate of reaction also increases because the gas molecules are closer together, so successful collisions are more frequent.
- However, the energy costs increase when higher pressures are used and the equipment becomes more expensive. Therefore, the choice of pressure is a compromise between yield and cost.
The Effect of Increasing Temperature
- When the temperature is increased, the position of equilibrium moves in the endothermic direction to reduce the temperature.
- In the Haber process, the forwards reaction is exothermic, so the reverse reaction is endothermic.
N2(g) + 3H2(g) ⇌ 2NH3(g) (forwards reaction is exothermic) - This means that as the temperature is increased, the position of equilibrium moves to the left, and the yield of ammonia decreases.
- It may seem sensible to use a very low temperature in order to maximise the yield of ammonia but lower temperatures reduce the rate of reaction. The temperature chosen is a compromise between yield and rate.
The Effect of Using a Catalyst
- A catalyst speeds up the rate of the forward and reverse reactions equally. This reduces the time taken for the system to reach equilibrium but it does not affect the position of equilibrium or the yield of ammonia.
- Using a catalyst in the Haber process means that a lower temperature can be used whilst keeping the rate of reaction high. A lower temperature helps to keep the yield high.
Reducing costs in other ways
- Most of the hydrogen and nitrogen which go into the reactor leave unreacted. By recycling them back into the reactor, the cost of making the reactants from raw materials is reduced.
- Energy is a significant cost for any chemical industry. Where reactions are exothermic and therefore release energy, this heat is often used to heat up other parts of the process. It can also sometimes be used to generate steam which is passed through a turbine connected to a generator in order to make electricity.
Choosing Reaction Conditions
Higher Tier Only
Economic Considerations
- Like all industries, companies that manufacture and sell chemical goods do so to make a profit
- Part of the industrial process is the economic decision on how and where to design and implement a manufacturing site
- The availability and cost of raw materials is a major consideration which must be studied well before any decisions are taken
- In the Haber Process the raw materials are readily available and inexpensive to purify:
- Nitrogen - from the air
- Hydrogen- from natural gas
- If the cost of extraction of raw materials is too high or they are unavailable then the process is no longer economically viable
- Many industrial processes require huge amounts of heat and pressure which is very expensive to maintain
- Production energy costs are also a factor to be considered carefully and alongside the raw materials issue
Temperature: 450ºC
- A higher temperature would favour the reverse reaction as it is endothermic (takes in heat) so a higher yield of reactants would be made
- If a lower temperature is used it favours the forward reaction as it is exothermic (releases heat) so a higher yield of products will be made
- However at a lower temperature the rate of reaction is very slow
- So 450ºC is a compromise temperature between having a lower yield of products but being made more quickly
Pressure: 200 atm
- A lower pressure would favour the reverse reaction as the system will try to increase the pressure by creating more molecules (4 molecules of gaseous reactants) so a higher yield of reactants will be made
- A higher pressure would favour the forward reaction as it will try to decrease the pressure by creating fewer molecules (2 molecules of gaseous products) so a higher yield of products will be made
- However, high pressures can be dangerous and very expensive equipment is needed
- So 200 atm is a compromise pressure between a lower yield of products being made safely and economically
 Choosing the conditions for the Haber Process
Choosing the conditions for the Haber Process
Catalyst
- The presence of a catalyst does not affect the position of equilibrium but it does increase the rate at which equilibrium is reached
- This is because the catalyst increases the rate of both the forward and backward reactions by the same amount (by providing an alternative pathway requiring lower activation energy)
- As a result, the concentration of reactants and products is nevertheless the same at equilibrium as it would be without the catalyst.
- So a catalyst is used as it helps the reaction reach equilibrium quicker
- It allows for an acceptable yield to be achieved at a lower temperature by lowering the activation energy required
- Without it the process would have to be carried out at an even higher temperature, increasing costs and decreasing yield as the higher temperature decomposes more of the NH3 molecules
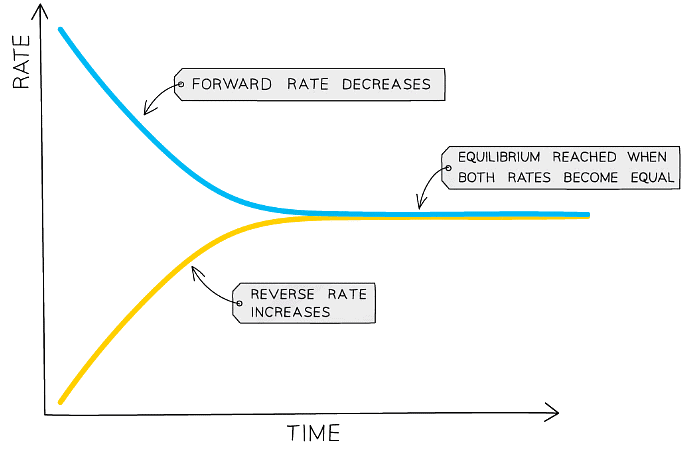 Diagram showing the effect of catalyst on equilibrium position
Diagram showing the effect of catalyst on equilibrium position
Exam Tip
The reaction conditions chosen for the Haber process are not ideal in terms of the yield but do provide balance between product yield, reaction rate and production cost. These are called compromise conditions as they are chosen to give a good compromise between the yield, rate and cost.
The document The Haber Process & Dynamic Equilibrium | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















